Abstract
Benign and malignant proliferations of histiocytes and dendritic cells may be encountered in lymph nodes. Reactive histiocytic and dendritic cell infiltrates occur in response to diverse stimuli and in addition to causing lymphadenopathy, may be present unexpectedly in lymph nodes excised for other indications. This review summarizes the pathogenesis and histopathological features of the various non-neoplastic histiocytic and dendritic cell infiltrates that can occur in lymph nodes.
Introduction
Histiocytes and dendritic cells have a specialized role in antigen presentation and in the phagocytosis and removal of cellular debris and pathogens. Accumulations of these cells in lymph nodes, therefore, are often seen as part of a reactive immune response to foreign material, infection or other antigens. Benign histiocytic proliferations in lymph nodes are much more commonly encountered than their malignant counterparts. Whilst an underlying cause for a reactive histiocytic infiltrate is not always apparent on histopathological analysis, in certain situations the histological features are characteristic and may indicate a possible etiology. This review discusses the histological features, pathogenesis and differential diagnosis of the reactive, non-neoplastic histiocytic and dendritic cell proliferations that may be encountered in lymph nodes in practice.
Origin and Functions of Histiocytes and Dendritic Cells
The modern concept of the mononuclear phagocyte system commenced in the late 1960s and was based on the principle that macrophages were derived from peripheral blood monocytes, which in turn were bone-marrow derived [1, 2]. The dendritic cell was subsequently discovered in 1973 [3]. These individual cell types have been characterized based on morphology, function and phenotype. Macrophages are large cells with abundant cytoplasm and a primarily phagocytic function. Dendritic cells have a stellate appearance and present antigen to naïve T-cells on MHC molecules [4–6]. The term ‘histiocyte’ has been variously used to describe tissue macrophages [2, 4] or both macrophages and dendritic cells [7].
Macrophages are tissue resident cells, whereas subsets of dendritic cells migrate to the lymph nodes from the peripheral tissues [8]. The Langerhans cell, a specialized dendritic cell, migrates from the epidermis and mucosal surfaces to the lymph nodes upon encountering antigen [9]. The lymph nodes also have a resident population of classical or myeloid dendritic cells, in addition to plasmacytoid dendritic cells [8]. Plasmacytoid dendritic cells secrete large amounts of type I interferons in response to the recognition of certain nucleic acid sequences [10, 11]. They also have the capacity to present antigen and can exert a tolerogenic or immunogenic effect on the immune response [11].
In recent years and mainly in mouse models, significant progress has been made in our understanding of the developmental pathways of macrophages, monocytes and dendritic cells. It is now known that adult macrophages and Langerhans cells have an embryonic origin and self-renew in the tissue independently from monocytes [9, 12, 13]. Dendritic cells (classical and plasmacytoid) and monocytes derive from bone marrow hematopoietic stem cells by way of distinct precursor pathways [6, 8]. Monocyte-derived cells may replenish populations of macrophages and dendritic cells at specific sites or under certain inflammatory conditions [12, 13]. Further studies will precisely delineate the relationship between these cells and the similarities and applicability of the findings in the mouse to human dendritic and monocyte cell subsets.
In addition to dendritic cells of hematopoietic origin, lymph nodes also contain mesenchymally-derived cells including follicular dendritic cells and fibroblastic reticulum cells [14, 15]. Follicular dendritic cells are associated with the B-cell follicles, where they maintain the follicular structure and organization of the germinal center and present antigen to B-cells [16, 17]. Fibroblastic reticular cells form an interconnected network that provides structure and organization to the lymph node. They are heterogeneous and comprise a number of subsets which support the growth of and regulate the migration of different cells within the lymph node through cellular interactions and chemokine secretion [15, 18, 19].
Some of the immunohistochemical stains that are used in routine practice to identify and differentiate between the major histiocytic and dendritic cell subsets in lymph nodes are listed in Table 1.
Table 1.
| Antibody | Macrophage | Langerhans cell | Interdigitating dendritic cell | Follicular dendritic cell | Plasmacytoid dendritic cell |
|---|---|---|---|---|---|
| CD68 | + | +/− Golgi | +/− | − | + |
| CD163 | + | − | − | − | − |
| S100 | −/+ | + | + | −/+ | − |
| Langerin | − | + | − | − | − |
| CD1a | − | + | − | − | − |
| CD21 | − | − | − | + | − |
| CD35 | − | − | − | + | − |
| CD123 | − | − | − | − | + |
| TCL1 | − | − | − | − | + |
| CD4 | + | + | + | + | + |
Histiocytic (Macrophage) Infiltrates
Sinus Histiocytosis
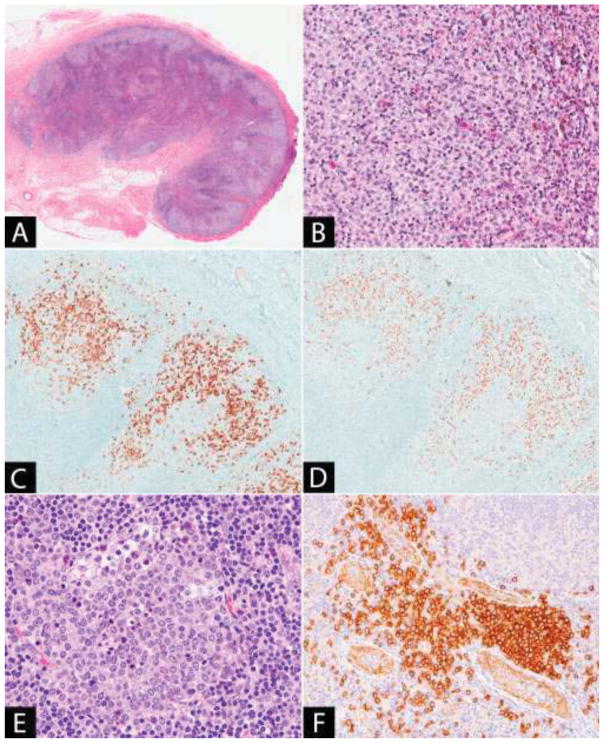
Sinus histiocytosis is a common feature in lymph node biopsies and is characterized by dilated lymph node sinuses containing variable numbers of histiocytes, with bland, indented nuclei and eosinophilic cytoplasm (Figure 1A). Many descriptions of sinus histiocytosis in the literature pertain to its prognostic significance in lymph nodes draining sites of tumor. The significance of this association varies, although a more favorable prognosis has been reported [20–22]. On occasion, the histiocytes may have a signet ring appearance, which may be mistaken for metastatic signet ring carcinoma or melanoma; however, the distinction is easily made by immunohistochemistry and special stains, as the histiocytes are positive with CD68 and negative for cytokeratin, S100 and mucin stains, although they may contain Periodic Acid-Schiff (PAS) positive globules [23–25].
Figure 1. (A) Sinus histiocytosis.
Lymph node sinuses are dilated and filled with benign-appearing histiocytes. (B–C) Hemophagocytic lymphohistiocytosis: (B) Histiocytes in the lymph node sinuses show prominent hemophagocytosis, (C) which can be highlighted using histiocytic markers (CD4 in image).
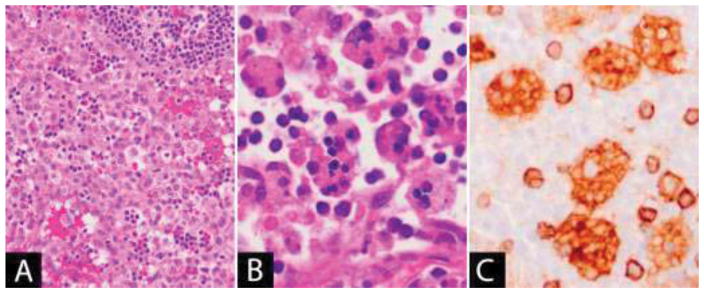
Hemophagocytic Lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) is an immune disorder characterized by systemic inflammation and uncontrolled hypercytokinemia. It is an uncommon [26–28] and life-threatening condition [29–31]. HLH presents with sudden onset fever, hepatosplenomegaly, generalized lymphadenopathy, malaise and cytopenias. The diagnostic criteria for HLH were updated in 2004 and require either a molecular diagnosis or 5 of 8 of the following clinical or laboratory parameters: (1) Fever, (2) Splenomegaly, (3) Cytopenias affecting 2 or more lineages, (4) Hypertriglyceridemia and/or hypofibrinogenemia, (5) Hemophagocytosis in bone marrow, spleen or lymph nodes, (6) Low or absent NK-cell activity, (7) Ferritin ≥500μg/L and (8) Soluble CD25 ≥2400U/mL [32].
HLH may be primary (familial) or secondary (acquired). Familial HLH (FHL) usually presents in the first year of life and results from mutations that interfere with the cytotoxic function of lymphocytes and NK-cells by affecting the formation of perforin or the trafficking, docking or membrane fusion of cytotoxic granules [33, 34]. In an effective cytotoxic response, lysis of the target cell removes the antigenic stimulus and terminates the inflammatory response. In HLH, it is thought that the defect in the cytotoxic pathway results in persistence of the stimulus and an inability to terminate the inflammatory response, leading to hypercytokinemia and tissue infiltration by macrophages, NK-cells and T-cells [28, 33, 35]. Mutations have been found in multiple genes in the cytotoxic pathway including: PRF1 (perforin) [36], UNC13D (Munc13-4) [37], STX11 (Syntaxin 11) [38] and STXBP2 (Munc18-2) [39, 40]. Certain mutations are associated with immunodeficiency or other manifestations such as hypopigmentation, including mutations in RAB27A, resulting in Griscelli syndrome type 2 [41], CHS1/LYST causing Chediak-Higashi syndrome [42, 43] and AP3B1 causing Hermansky-Pudlak syndrome type 2 [34, 44, 45]. X-linked lymphoproliferative disease and several primary immunodeficiencies also predispose to HLH or HLH-like manifestations usually mediated by Epstein-Barr virus (EBV) infection [4, 28, 46, 47] (Table 2). Acquired HLH is due to secondary causes including infection (EBV, Herpes viruses), malignancy and rheumatological conditions, where it is also referred to as macrophage activation syndrome (MAS) [4, 28, 48, 49]. The mechanisms of acquired HLH are still unclear, but hypercytokinemia is a common thread [33, 35].
Table 2.
| Disease | Chromosome | Gene | Protein | Function | Associated Features |
|---|---|---|---|---|---|
| FHL1 [201] | 9q21.3-22 | Unknown | Unknown | Unknown | |
| FHL2 [36] | 10q21-22 | PRF1 | Perforin | Creates pores in target cell | |
| FHL3 [37] | 17q25 | UNC13D | Munc13-4 | Vesicle priming preceding vesicle membrane fusion | |
| FHL4 [38] | 6q24 | STX11 | Syntaxin11 | Vesicle membrane fusion | |
| FHL5 [39, 40] | 19p13 | STXBP2 | Munc18-2 | Vesicle membrane fusion | Severe diarrhea |
| Griscelli syndrome, type 2 [41] | 15q21 | RAB27A | Rab27a | Small GTPase family of proteins – vesicular docking | Partial albinism |
| Chediak-Higashi syndrome [42, 43] | 1q42.1-42.2 | LYST | LYST (lysosomal trafficking regulator) | BEACH family of proteins. Vesicle trafficking regulatory function. | Partial albinism, immunodeficiency, leukocyte giant granules, neurological disorder |
| Hermansky-Pudlak syndrome, type 2 [44, 45] | 5q14.1 | AP3B1 | Subunit β3A of AP-3 complex | Lysosomal trafficking | Oculocutaneous albinism, bleeding diathesis, neutropenia, recurrent infections |
| X-linked lymphoproliferative disease, type 1 [46] | Xq25 | SH2D1A | SAP (SLAM- associated protein) | Cell signaling | Fulminant infectious mononucleosis, EBV-related HLH, lymphoid proliferations, hypogammaglobulinemia, risk of lymphoma |
| X-linked lymphoproliferative disease, type 2 [47] | Xq25 | BIRC4 | XIAP (X-linked inhibitor of apoptosis) | NFκB and MAPK pathways, inhibitor of apoptosis | HLH (EBV, CMV or HHV-6 infection), hypogammaglobulinemia, inflammatory bowel disease |
| Other primary immunodeficiencies [4, 28] | CD27 deficiency - CD27 EBV-associated autosomal lymphoproliferative syndrome (ITK deficiency) - ITK X-linked immunodeficiency with magnesium defect, EBV infection and neoplasia (XMEN) - MAGT1 |
EBV-associated HLH/HLH-like manifestations; lymphoproliferation, immunodeficiency | |||
The characteristic histopathological feature is hemophagocytosis, the phagocytosis of other hematopoietic cells by the activated macrophages that infiltrate the tissue (Figure 1B–C). The preferred diagnostic material is usually a bone marrow aspirate; however, hemophagocytosis may be observed in other tissues including lymph nodes, spleen and liver [35, 49]. Lymph node histology in HLH can be variable. The macrophages are cytologically benign and may diffusely infiltrate the nodal parenchyma or involve the nodal sinuses [50, 51]. In cases of HLH due to underlying EBV infection, the predominant feature may be an immunoblastic reaction with demonstrable EBV [51]. Immunohistochemistry with histiocytic markers (CD68, CD163 or others) is helpful in identifying the phagocytic macrophages (Figure 1C). The macrophages may show variably positive staining for S100, in contrast to the emperipoletic histiocytes of Rosai-Dorfman disease which are strongly positive [50, 52]. In patients with a perforin deficiency, perforin can be absent in the cytotoxic lymphocytes by flow cytometry or immunohistochemistry [53, 54]. HLH may also occur in the context of an underlying malignancy, particularly lymphoma or leukemia; therefore, such processes should be sought when examining the lymph node biopsy. The diagnosis of HLH is a clinical one, as hemophagocytosis is not entirely sensitive or specific: it is not always present on biopsy in the setting of HLH, and furthermore, it can be seen in the tissue in the absence of HLH, such as in the setting of sepsis or surgery [30, 35, 55, 56].
Whipple’s Disease
Whipple’s disease is a rare multisystem illness caused by the rod-shaped bacillus Tropheryma whipplei. Although initially described by George Hoyt Whipple in 1907 [57], the first case of the disease was probably reported earlier by Allchin and Hebb in 1895 [58, 59]. The pathogenic organism remained unknown until the 1990s [60]. T. whipplei is genetically diverse, but the strain of the organism has not been found to correlate with the clinical presentation of the infection [61, 62]. The classic form of the disease typically affects middle-aged white men and involves the intestinal tract, with symptoms of fever, abdominal pain, weight loss and diarrhea [58, 63–65]. Abdominal lymphadenopathy is often present. Arthralgia is also common, in particular it manifests as a prodromal stage preceding the development of gastrointestinal symptoms. The disease may affect the central nervous system and heart [64, 65]. Peripheral lymphadenopathy is present in approximately 50% [63, 66, 67]. In addition to the classic form, T. whipplei may cause an acute infection [65, 68] or infection localized to extra-intestinal sites [65]. Asymptomatic carriage of the organism also occurs and it may be detected in saliva and stool [64, 69].
Classic Whipple’s disease is rare, with an estimated incidence of between 1 and 6 new cases per 10,000,000 persons per year worldwide [65]; however, the higher asymptomatic carrier rates suggest that genetic predisposition or immune dysfunction are important factors in the pathogenesis of the disease. Studies have found an increased frequency of HLA DRB1*13 or DQB1*06 in patients with Whipple’s disease [70], and have also demonstrated abnormalities in macrophage function and T-cell responses. Macrophages in patients with Whipple’s disease have been shown to be shifted towards an alternatively activated phenotype with high expression of IL-10 and reduced IL-12 expression [64, 71–73]. There is skewing towards Th2 and T-regulatory cell responses, and a reduced Tropheryma whipplei-specific Th1 response has been found in patients with Whipple’s disease [74–78]. IL-16 has been implicated in macrophage phagosome dysfunction and bacterial replication [79, 80].
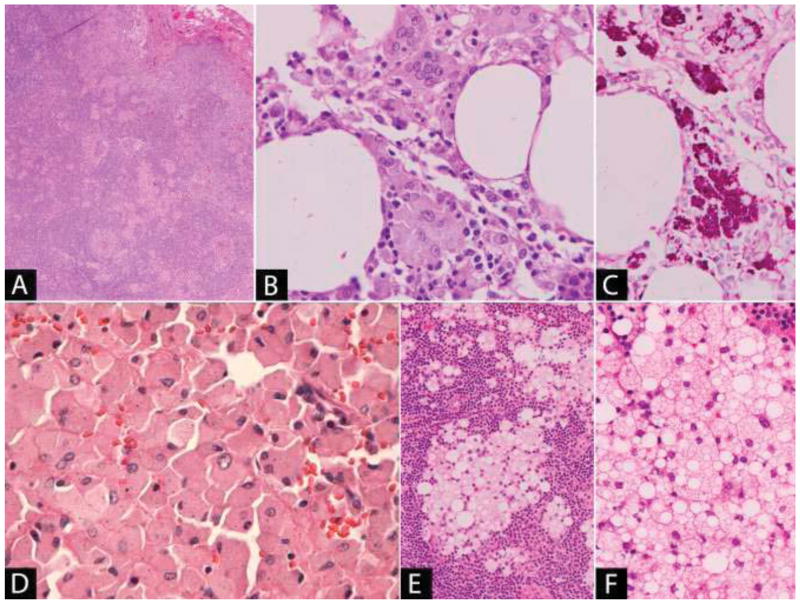
Histopathological diagnosis is usually made on duodenal or proximal small bowel biopsies which show aggregates of foamy macrophages present in the lamina propria. Involved lymph nodes may show capsular fibrosis with dilated cystic spaces and nodal sinuses [66]. The characteristic histiocytes can have a sinusoidal or paracortical distribution. Foreign body giant cells and epithelioid granulomas may be present; however, necrosis is typically absent (Figure 2A–B) [81–83]. The macrophages are positive with CD68 and the intracellular bacteria are PAS-positive [84], diastase resistant (Figure 2C). Ziehl-Neelson (ZN) stain is negative, allowing distinction from mycobacterial infection. The diagnosis can be confirmed by T. whipplei specific PCR or by specific immunohistochemistry [64, 81]. Asymptomatic carrier states of T. whipplei may occur in the gastrointestinal and respiratory tracts, so a positive PCR result from these sites necessitates correlation with additional parameters [64, 81]. The presence of epithelioid granulomas in nodal sites may mimic sarcoidosis, a pitfall documented in several reports [82, 83, 85]. Of note in these cases, the PAS stain may be negative and the intracellular bacteria present in low numbers by electron microscopy.
Figure 2. (A–C) Whipple’s disease.
(A) Small aggregates of macrophages are present in the lymph node sinuses and in the parenchyma forming loose epithelioid granulomas. (B) The macrophages have eosinophilic granular cytoplasm and occasional multinucleated giant cells are present. (C) The bacilli are positive with DPAS stain. (D) Histiocytosis following joint replacement: Sheets of polygonal histiocytes with abundant eosinophilic cytoplasm containing small black particles consistent with metal wear debris. (E–F) Silicone lymphadenopathy due to breast implant: (E) Aggregates of macrophages with abundant foamy, vacuolar cytoplasm are present within the lymph node. (F) The vacuoles are colorless and the silicone, where present, has a refractile quality and is non-polarizable.
Treatment is with antibiotic therapy and the disease is ultimately fatal if not treated [58, 63, 64]. PAS positive macrophages may persist for some time after therapy and improvement in clinical symptoms [86, 87]. Patients with Whipple’s disease may initially be misdiagnosed with a seronegative arthritis and treated with immunosuppression. Such patients are at an increased risk of developing immune reconstitution inflammatory syndrome (IRIS) during antimicrobial therapy [88]. The disease may relapse after many years and life-long follow up of patients is warranted [78].
Reactive Histiocytosis following Prosthetic Arthroplasty
Arthroplasty or joint replacement is a common surgical procedure for treatment of damaged or diseased joints and articular surfaces due to degenerative joint disease, rheumatoid arthritis, malignancy or trauma. The components of an artificial joint may be manufactured from metals such as stainless steels and cobalt-chrome, ultrahigh molecular weight polyethylene or ceramic. The prosthesis may be fixed in place using a polymethylmethacrylate cement. Usually, both articular surfaces of a joint are replaced, and the replacement surfaces may be composed of similar or different materials [89]. Histiocytic infiltrates can occur in lymph nodes draining the sites of arthroplasty prostheses in response to wear particles that are generated from the interface between the individual artificial joint components, as well as the adjacent bone [90–92]. Over time, interaction or wear at the sites of the replaced articular surfaces leads to the production and deposition of these particles in the periarticular tissue initiating a local inflammatory response with cytokine release [90, 92–95]. Wear particles may drain from the soft tissue to regional lymph nodes as free particles which are phagocytosed by macrophages forming nodal aggregates. It is also possible that some phagocytosis of the particles may occur in the periarticular tissue by macrophages which subsequently enter the lymphatics [90, 91, 95].
Histologically, involved lymph nodes show sinusoidal and interfollicular expansion by sheets of polygonal histiocytes with abundant granular or foamy cytoplasm and round nuclei, without cytologic atypia. Foreign-body type giant cells may also be present (Figure 2D) [90, 91, 93–97]. The microscopic characteristics of the wear particles depend on the material used in the prosthesis. Metal wear debris may be identified as small, irregular black particles within the cytoplasm of the histiocytes and sometimes extracellularly [90, 95, 96, 98]. Occasionally, metal particles may be numerous and produce tattooing; however, the particles can also be subtle and may be overlooked in the setting of a florid reactive histiocytic proliferation [90, 96]. The presence of metals including cobalt-chromium, titanium, molybdenum or iron in affected lymph nodes has been confirmed using energy dispersive x-ray microanalysis [90, 96] and inductively coupled plasma mass spectrometry [98].
Polyethylene wear fragments are colorless and vary in size. The particles are present as needle-like shards within macrophages and multinucleated giant cells. Polyethylene is intensely birefringent under polarized light [90, 91, 93–95]. Polymethylmethacrylate (PMMA) dissolves during tissue processing; however, is usually admixed with particles of a radiodense metal (barium or zirconium oxide) and is therefore characterized histologically in the implant bed by a large clear space with surrounding histiocytes and giant cells containing scattered granules of the admixed metal [89, 93, 99]. It is not commonly described in lymph nodes as it is not visible on paraffin sections; however, it has been suggested that phagocytosed PMMA may contribute significantly to the cytoplasmic appearance of the histiocytes in some cases [97, 100].
Resulting lymphadenopathy may be of clinical concern, particularly when the context raises the possibility of metastatic disease, such as in patients with a prosthesis resulting from treatment of a bone malignancy or the onset of pelvic lymphadenopathy in a patient with gynecologic or genitourinary tract malignancy. The identification of the metal or polyethylene fragments should prompt clinical correlation in cases where the history of a prosthesis is unknown.
Silicone Lymphadenopathy
Silicone lymphadenopathy usually occurs in regional lymph nodes draining sites of silicone-containing medical implants, typically silicone gel-filled breast implants [101] or silicone elastomer joint prostheses [102]. As local lymph nodes are involved, the axilla is the usual site of adenopathy in cases associated with breast implants and prostheses used in the replacement of the small joints of the hand; however, silicone may also travel to more distant sites in the body and has been described in supraclavicular and cervical lymph nodes [103], and in lymph nodes in the contralateral axilla to the breast implant [104].
Silicone gel from breast implants may enter the lymphovascular channels due to implant rupture or from miniscule leakages or “bleeding” from an intact implant bag [101, 105–107]. In the case of an elastomer-containing joint prosthesis, small fragments of the elastomer may become detached from the surface of the prosthesis due to wear and enter the tissue [105]. The silicone used in medical implants is composed of dimethylsiloxane polymers and the nature of the silicone (liquid, gel or elastomer) depends on the length and cross-linking of the polymer chains [106, 108].
The extent of the nodal effacement ranges from focal involvement to the presence of substantial parenchymal infiltrates. The histological features of the process differ depending on the consistency of the silicone present in the lymph nodes [105, 106]. Silicone elastomer causes a foreign body giant cell reaction which is sometimes associated with the formation of non-necrotizing granulomas composed of epithelioid histiocytes [102, 109]. The silicone fragments are present within the cytoplasm of the giant cells or within the granulomatous inflammatory reaction, which on occasion, may be marked [110]. Silicone in a liquid or gel form causes vacuoles of different sizes within the nodal parenchyma. These silicone particles are taken up by histiocytes, giving them a characteristic foamy, heavily vacuolated cytoplasm [106, 107] (Figure 2E–F). The number of giant cells present is usually much fewer than is seen with silicone elastomer [105]. The silicone may not survive tissue processing, and therefore the vacuoles may be devoid of material. If present, silicone appears as strands of a refractile, non-polarizable, clear substance within the vacuoles [101, 106, 107]. Various methods have been used in different studies to confirm the presence of silicone in the lymph nodes including energy dispersive x-ray analysis [105, 106], confocal laser-Raman microprobe (CLRM) spectroscopy [107] and Fourier transform infrared spectroscopy (FTIR) [107, 111].
Silicone lymphadenopathy may clinically mimic a malignant process, such as lymphoma [112]. An important consideration is metastatic carcinoma, particularly in patients with a breast implant for reconstructive purposes following resection of a breast malignancy. Some reports have shown that the involved nodes may be PET scan positive, which may heighten the clinical concern for a malignant process [113, 114]. In such situations, the histological features may raise the consideration of a metastatic lobular carcinoma; however, the vacuolated cells will be positive for histiocytic markers such as CD68, and negative for cytokeratin and histochemical stains for mucin, confirming the benign reactive nature of the process.
Crystal Storing Histiocytosis
Crystal storing histiocytosis is a rare disorder characterized by aggregates of histiocytes with abnormal intracytoplasmic crystals. In most cases (90% in one review [115]), the histiocytosis occurs in association with a B-cell lymphoproliferative disorder, usually lymphoplasmacytic lymphoma [116, 117], multiple myeloma [118] or monoclonal gammopathy of uncertain significance (MGUS) [119]; however, it has also been described in inflammatory and autoimmune conditions including rheumatoid arthritis [120], H. pylori infection [121] and Crohn’s disease [122]. Crystal storing histiocytosis can be classified according to the disease association or the composition of the crystals [115]. In most cases, the crystals are composed of immunoglobulin, but crystal storing histiocytes have also been described with the use of the drug clofazimine [123] to treat leprosy, in hereditary cystinosis [124] and with Charcot-Leyden crystals in the setting of eosinophilic inflammation [125].
The mechanism by which the crystals accumulate within the histiocytes is not known, but it is thought to be due to the structural properties of the immunoglobulin in combination with high levels of the immunoglobulin in the serum [120, 126]. It has been suggested that conformational changes in the immunoglobulin due to amino acid substitutions may be a pathogenic factor, with one case report identifying unusual amino acid substitutions, including at a site important for hydrophobic interactions within the protein structure [127]. Inherited or acquired processing defects in histiocytes have also been theorized [128]. Ultrastructural studies performed indicate that the immunoglobulin is endocytosed by macrophages and the crystals are formed within lysosomes during lysosomal digestion [117, 129, 130]. In the case of clofazimine, the drug has been observed within macrophage phagosomes prior to the development of the crystals [123, 131].
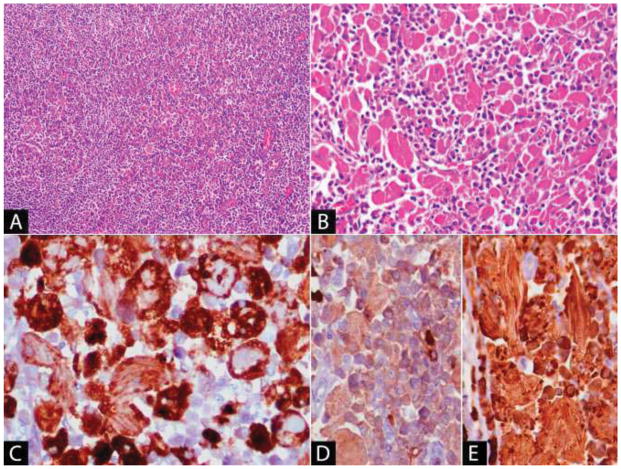
Histologically, the histiocytes are ovoid to spindle shaped, with abundant eosinophilic cytoplasm and contain packed elongated structures, some of which may show a parallel arrangement. The nuclear features are benign and the nuclei may be peripheralized within the cell (Figure 3). Occasional multinucleated cells may be present [116, 117, 120, 132, 133]. The histiocytic infiltrate is variable, but may be so prominent as to obscure an associated B-cell lymphoma [115, 128]. The histiocytes are positive for histiocytic markers such as CD68 (Figure 3C) and CD163 and are negative for desmin, S100 and markers of B-cell/plasma cell lineage [116, 120, 133]. Immunoglobulin crystals within the cytoplasm of the histiocytes may show monotypic light chain or heavy chain expression (Figure 3D–E); however, cases without demonstrable staining have been described. The negativity of the crystals in these cases has been attributed to the altered immunoglobulin structure or to poor tissue fixation [116]. There is no clear association with any individual heavy or light chain class [115]. Histochemically, immunoglobulin crystals stain blue with phosphotungstic acid hematoxylin, and show variable staining with PAS [116, 120, 132]. They are needle-shaped or rhomboid-shaped on ultrastructural examination [120, 129, 133]. Clofazimine crystals are red and show bright-red birefringence in frozen sections; however, they dissolve during tissue processing and are therefore colorless in formalin-fixed paraffin-embedded tissue sections [123].
Figure 3. Crystal storing histiocytosis associated with marginal zone lymphoma with plasmacytic differentiation.
(A) The lymphoma component is diffuse in areas, containing only scattered admixed histiocytes. (B) Elsewhere, there are sheets of histiocytes with abundant eosinophilic fibrillary cytoplasm. (C) The histiocytes are positive with CD68, which aids in visualizing the intracytoplasmic rhomboid and needle-shaped crystals. Immunohistochemistry for kappa (D) and lambda (E) show that the crystals and background plasma cells in this case are positive for lambda light chain.
The differential diagnosis of crystal storing histiocytosis includes rhabdomyoma[116], from which it can be distinguished by immunohistochemistry, malakoplakia and storage disorders such as Gaucher disease.
Lysosomal Storage Disorders
Lysosomal storage disorders are rare diseases, some of which are associated with histiocytic infiltrates in bone marrow and other organs including lymph nodes.
Niemann-Pick disease comprises two different abnormalities in lipid metabolism: types A and B are due to a functional deficiency of acid sphingomyelinase (ASM), whereas type C results from defective intracellular trafficking of cholesterol [134, 135]. In types A and B, many different mutations and deletions have been reported in the SMPD1 gene on chromosome 11p15.4, which encodes ASM [135]. Most patients with Niemann-Pick type C have mutations in the NPC1 gene on chromosome 18q11-q12 (approximately 95%), other patients have mutations in the NPC2 gene on chromosome 14q24.3 [134]. The Niemann-Pick cell is lipid-laden with abundant foamy cytoplasm containing vacuoles of sphingomyelin or cholesterol – the so-called ‘mulberry appearance’ [135].
Gaucher disease is an autosomal recessive sphingolipidosis caused by mutations in the glucocerebrosidase gene (GBA) on chromosome 1q21. Mutations reduce the enzymatic activity of β-glucocerebrosidase resulting in the accumulation of its substrate, glucosylceramide (glucocerebroside), in macrophage lysosomes. The characteristic Gaucher cells have abundant blue-grey cytoplasm with a fibrillary appearance that is often compared to wrinkled tissue paper [136, 137] and are positive with PAS and Prussian blue stains [136, 138]. Pseudo-Gaucher cells may be seen in association with other conditions including chronic myeloid leukemia and hemoglobinopathies. There are 3 major clinical phenotypes, the most common, type 1, is non-neuropathic and characterized by hepatosplenomegaly, cytopenias and bone marrow involvement. Types 2 and 3 both have neurological involvement, with type 2 usually fatal within the first 1–2 years of life [136, 137].
Granulomatous Lymphadenitis
Granulomatous inflammation is a form of chronic inflammation that occurs in response to infectious, autoimmune, neoplastic and unknown causes [139]. The granuloma is composed of an admixture of cells of the mononuclear phagocyte system, including epithelioid histiocytes and multinucleated giant cells. Epithelioid histiocytes form clusters and are characterized by abundant eosinophilic, granular cytoplasm, indistinct cell borders and oval or elongate nuclei. Multinucleated giant cells in granulomas were traditionally described as either Langhans type (with multiple nuclei organized at the periphery of the cell) or foreign-body type (nuclei present throughout the cytoplasm) [139, 140]. Both types can be present. Granulomatous inflammation occurs in response to an antigen that is insoluble or otherwise difficult to eliminate [139]. Macrophages enter the tissue and secrete TNF-α and pro-inflammatory cytokines including IL-12 and IL-23, recruiting other inflammatory cells and CD4-positive T-cells.
Typically, a Th1 immune response predominates with IFN-γ production resulting in granuloma formation, although the exact components of the granuloma vary with the underlying cause [140–142]. It has been suggested that a Th2 response may develop over time in certain autoinflammatory granulomata, with shifting of the macrophage phenotype from M1 to M2 as fibrosis develops [141, 142]. Macrophage polarization and diversity are also important in the response to Mycobacterium tuberculosis infection [143].
Histologically, granulomatous inflammation can be categorized as non-necrotizing, necrotizing and suppurative [50] (Table 3).
Table 3.
Differential Diagnosis of Granulomatous Inflammation in Lymph Nodes [50]
| Necrotizing/Suppurative Granulomas | Clinical | Nodal Sites | Histological Features1 | Histochemical stains & Immunohistochemistry | Other Investigations |
|---|---|---|---|---|---|
| Mycobacterial lymphadenitis (Mycobacterium tuberculosis) [153, 154] | History of exposure to TB | Cervical, axillary, mediastinal | Central necrosis. Acid fast bacilli |
Ziehl-Neelson: + Fite: + |
Tuberculin skin test IFN-γ release assay PCR/culture |
| Histoplasmosis (Histoplasma capsulatum) [156, 157] | Asymptomatic, pulmonary infection or disseminated depending on immune status and exposure level | Hilar or others in disseminated | 2–4μm, clustered in macrophages. Difficult to appreciate in granulomas without histochemical stains |
GMS: + PAS: + Mucicarmine: − |
Serology Histoplasma antigen EIA Culture |
| Cryptococcosis (Cryptococcus neoformans, Cryptococcus gattii) [156, 202, 203] | Pulmonary involvement. May involve CNS. May be disseminated |
Cervical, hilar, axillary, inguinal and others | Well-formed granulomas. May form cystic spaces with gelatinous material. 5–10μm - within clear spaces in granulomas and macrophages | PAS: + GMS: + Mucicarmine: + Fontana-Masson: + |
Cryptococcal antigen EIA Culture |
| Cat Scratch disease (Bartonella henslae) [158, 159, 162, 163] | History of contact with cats Skin lesion |
Axillary, cervical, epitrochlear | Warthin-Starry: Pleomorphic coccoid or curved bacilli in clumps or singly in vessel walls or in foci of necrosis | Brown-Hopps: Faint (gram- negative) Warthin-Starry: + Ziehl-Neelson: − Immunohistochemistry for Bartonella henslae |
Serology PCR |
| Lymphogranuloma venereum (Chlamydia trachomatis, L1, L2, L3) [164, 204] | Mucosal or skin lesions in genital tract or rectum | Inguinal, femoral or iliac nodes. May fistulate. | Macrophages with vacuoles. Organisms form a central clump or a peripheral rim in vacuoles | Warthin-Starry: + PAS: − Ziehl-Neelson: − Brown-Hopps: Gram- negative |
Serology Nucleic acid detection |
| 1. H&E. Features on special stain if indicated | |||||
| Non-Necrotizing Granulomas | Clinical | Nodal Sites | Histological Features | Histochemical stains & Immunohistochemistry | Other Investigations |
| Sarcoidosis [142, 144, 148] | Multi-system disease; pulmonary symptoms; erythema nodosum | Bi-hilar adenopathy | Sharply demarcated granulomas; Asteroid bodies, Schaumann bodies; Hamazaki- Wesenberg bodies | Ziehl-Neelson and fungal stains are negative. Hamazaki-Wesenberg bodies are GMS + and PAS + (Pitfall) |
Serum ACE levels Chest x-ray Microbiological cultures |
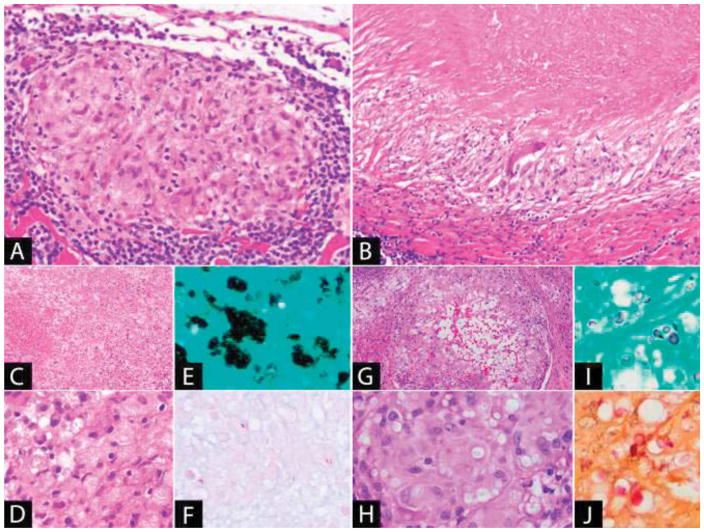
The prototypical example of non-necrotizing granulomatous inflammation (Figure 4A) occurs in sarcoidosis, a multi-system disease in which the underlying cause is still unknown [142, 144]. Histologically, sarcoidal granulomas are well-demarcated, usually numerous and in close proximity to one another in involved lymph nodes. They contain both epithelioid cells and giant cells, and may have focal necrosis [145, 146]. Cytoplasmic inclusions can be identified within the granulomas, including asteroid bodies (spiculated inclusions in giant cells composed of complex lipoproteins) and Schaumann bodies (calcified structures with concentric lamellations) [145, 147, 148]. Hamazaki-Wesenberg bodies are thought to represent extracellular giant residual lysosomal bodies [149] and may be present near the subcapsular sinuses [146]. They are positive with Gomori methenamine silver (GMS) and PAS stains, and therefore present a pitfall in that they may be mistaken for fungal infection [150]. These inclusions, whilst commonly described in association with sarcoidosis, are not specific and occur in other conditions. Non-necrotizing granulomata can also be present in lymph nodes in Crohn’s disease [151] and a granulomatous reaction with or without necrosis may also occur in lymph nodes in association with malignancy, including carcinoma, Hodgkin’s lymphoma and non-Hodgkin’s lymphoma [152].
Figure 4. Granulomatous Inflammation.
(A) Non-necrotizing granuloma: The granuloma is well-demarcated and composed of epithelioid histiocytes with a peripheral lymphoid cuff. (B) Necrotizing granuloma: There is a large area of necrosis surrounded by a peripheral rim of epithelioid histiocytes. A giant cell is also present. (C–E) Disseminated histoplasmosis: (C) The lymph node is effaced by sheets of macrophages with extensive necrosis. (D) The yeast is present in clusters within the cytoplasm of the macrophages and is positive with (E) GMS stain. (F) Acid-fast bacilli (Fite): Mycobacteria, a cause of necrotizing granulomatous lymphadenitis, may be few in number and difficult to identify. (G–J): Cryptococcal lymphadenitis: (G) Granulomatous inflammation with central cystic spaces. (H) The yeast is visible within the cytoplasm of the histiocytes as faintly staining structures within clear spaces. It is positive with (I) GMS and (J) mucicarmine stains.
Necrotizing granulomas (Figure 4B) usually occur due to an infectious process and are characteristically seen with Mycobacterium tuberculosis infection, in which cervical lymphadenopathy is the most common site of peripheral lymph node involvement [153]. Tuberculous granulomas are composed of a central area of necrosis surrounded by epithelioid histiocytes, Langhans-type giant cells and lymphocytes. The central necrosis typically does not contain cellular debris. With age, fibrosis and calcification of the lymph node occurs [154]. Mycobacterial organisms are acid-fast and can be demonstrated using ZN or Fite stains (Figure 4F), although demonstration of the organism is often unsuccessful in tissue sections and bacterial culture or PCR are used to identify the organism [153, 155]. Fungal infection may also result in a necrotizing granulomatous lymphadenitis, such as occurs in Histoplasma capsulatum infection. Lymph nodes involved by H. capsulatum contain epithelioid granulomas and sheets of yeast-containing macrophages which undergo necrosis (Figure 4C). The yeast is round to oval, 2–4μm in size and visible in macrophages as intracytoplasmic clusters (Figure 4D–E). It is difficult to appreciate in the epithelioid granulomas on H&E; however, is detectable with GMS stain [156, 157]. Histoplasma capsulatum must be distinguished from Cryptococcus (Figure 4G–J), which in addition to GMS, also stains with mucicarmine and Fontana-Masson stains [156].
Suppurative granulomatous inflammation is seen in Cat Scratch disease, a usually self-limited lymphadenitis caused by the gram-negative bacterium Bartonella henslae. Histologically, the lymph node shows stellate-shaped neutrophilic abscesses, with a surrounding rim of epithelioid histiocytes and Langhans-type giant cells. In the early stages of involvement, there may be follicular hyperplasia and a monocytoid B-cell reaction [158–160]. The organism can be demonstrated using a Warthin-Starry stain as pleomorphic bacilli present singly or in clumps in the foci of necrosis, in macrophages, or in the walls of capillaries [161]. Immunohistochemical stains are also useful, as they avoid the background precipitate that may make interpretation of silver stains difficult [162] and PCR can also identify the organism in formalin-fixed tissue [163]. Other causes of suppurative granulomatous inflammation include lymphogranuloma venereum (Chlamydia trachomatis L1, L2, L3) [164] and tularemia (Francisella tularensis) [165].
Mixed Histiocytic and Dendritic Cell Infiltrates
Dermatopathic Lymphadenopathy
Dermatopathic lymphadenopathy is a reactive condition that classically occurs in lymph nodes draining sites of chronic skin disorders. Some of the earliest descriptions have been variously attributed in the literature to Jadasson in 1892 [166, 167], Wise in 1917 [168, 169] or to Pautrier and Woringer in French (réticulose lipo-mélanique/lipomelanotic reticulosis) in the 1930s [169–171]. The process was eventually termed dermatopathic lymphadenitis in 1942 by Hurwitt [167]. The lymphadenopathy may be generalized or localized. Axillary and inguinal lymph nodes [169, 171] are most frequently involved and a peripheral blood eosinophilia may be present [166, 167, 169, 172].
Dermatopathic lymphadenopathy occurs at any age, but it is more common in the 5th and 6th decades [169, 173] and in males [166, 167, 169, 172]. In their series of 906 consecutive lymph node biopsies, Cooper et al reported an incidence of 4.8% with 15% (6/40) of their cases of dermatopathic lymphadenopathy occurring in patients with mycosis fungoides [169]. Other skin conditions described in patients with dermatopathic lymphadenopathy include psoriasis, eczema, exfoliative dermatitis, neurodermatitis, cutaneous hypersensitivity reactions, seborrheic dermatitis and non-specific chronic dermatitis [166, 167, 169].
Whilst theories regarding the pathogenesis of dermatopathic lymphadenopathy have centered on an inflammatory reaction with excessive absorption of melanin from the skin due to the underlying skin disorder [166, 167, 170, 172], some lymph nodes may have histologic features of dermatopathic lymphadenopathy in the absence of an associated skin disease [169]. Gould et al, in a study of 1181 lymph nodes from axillary dissections from patients without skin disease, observed varying degrees of dermatopathic-like histologic features, concluding that dermatopathic lymphadenopathy “may represent one end of a normally occurring histologic spectrum that may be found in the absence of a dermatitis” [170, 174]. There is also the consideration that changes may reflect an undocumented prior dermatitis [174] - in one study, a lapse of 40 years was reported in one case between the resolution of skin changes and the lymph node biopsy [166].
Histologically, the architecture of the lymph node is intact, but there is variable expansion of the paracortex by a pale staining proliferation composed of interdigitating dendritic cells, histiocytes and Langerhans cells, which may compress and peripheralize the cortical areas of the node [166, 174, 175] and surround residual atrophic follicles [172]. Mitotic activity may be present. At the margins of the proliferation [166, 169], some of the histiocytes contain phagocytized pigment, usually melanin, although some hemosiderin may be present [167, 169]. Phagocytized cytoplasmic lipid may also be evident [169, 172]. The infiltrate contains variable numbers of lymphocytes, plasma cells and eosinophils (Figure 5A–B).
Figure 5. (A–D) Dermatopathic lymphadenitis.
(A) The lymph node paracortex is expanded by pale staining nodules. (B) The pale staining areas are composed of an admixture of dendritic cells, Langerhans cells and histiocytes, some of which contain pigment. The Langerhans cells may be numerous and are identified by immunohistochemistry with (C) CD1a and (D) langerin. (E–F) Plasmacytoid dendritic cell aggregate: (E) The plasmacytoid dendritic cells have pale cytoplasm and fine chromatin. Scattered tingible body macrophages are present. (F) CD123 stains both the plasmacytoid dendritic cells and the endothelial cells, highlighting the location of the plasmacytoid dendritic cell aggregate adjacent to the high endothelial venules.
By immunohistochemistry, the interdigitating dendritic cells are positive for S100 and negative for CD1a and langerin. Langerhans cells are positive for S100, CD1a and langerin (Figure 5C–D) [175, 176]. The paracortical pattern of the infiltrate is useful in the distinction from Langerhans cell histiocytosis, which is generally sinusoidal. The associated histiocytes are positive for CD68 and lysozyme and the presence of melanin and hemosiderin can be confirmed by histochemical stains [167, 169, 177].
As dermatopathic lymphadenopathy occurs in lymph nodes from patients with mycosis fungoides and Sézary Syndrome, a well-recognized diagnostic difficulty lies in the assessment of early nodal involvement by the T-cell malignancy in dermatopathic lymph nodes. Several studies have examined histologic, immunophenotypic and electron microscopy parameters to distinguish between subtle early nodal involvement by mycosis fungoides and dermatopathic lymphadenopathy without involvement by lymphoma; however, no characteristic feature has been found that can definitively discriminate between the two processes using these methods [170, 173, 174, 178, 179]. Molecular studies for T-cell receptor gene rearrangements have proved useful in identifying monoclonal T-cell populations in dermatopathic lymph nodes from patients with mycosis fungoides in the absence of overt histological involvement, suggesting the diagnosis of early nodal involvement by lymphoma. The presence of molecular lymph node involvement in this group of patients may predict a poorer prognosis [180, 181].
Other Dendritic Cell Proliferations
Plasmacytoid Dendritic Cell Proliferations
Plasmacytoid dendritic cells (PDCs) are a normal cellular component of reactive lymph nodes; however, increased numbers and clusters have been reported in certain conditions including hyaline-vascular Castleman disease, histiocytic necrotizing lymphadenitis, Kimura disease [182] and granulomatous lymphadenitis [183]. Histologically, the PDCs are recognizable as clusters of medium-sized cells with fine chromatin and pale cytoplasm. The aggregates have interspersed tingible body macrophages, and are situated in the paracortex, usually in proximity to the high endothelial venules (Figure 5E) [184]. PDCs are identifiable by immunohistochemistry (Figure 5F), as the cells are positive for CD123, BDCA2(CD303), granzyme B and TCL-1 and negative for specific markers of B-cell, T-cell and myeloid lineages. CD2AP and CD4 are often expressed, and granular staining for CD68 may be seen. They are negative for CD34 and TdT [184, 185].
Mature plasmacytoid dendritic cell proliferations are also associated with myeloid neoplasms, mainly chronic myelomonocytic leukemia, but also with myelodysplasia and acute leukemia with monocytic differentiation [185, 186]. The proliferations occur in the lymph nodes, skin and bone marrow and may be significant, comprising numerous, large nodules of PDCs with conspicuous apoptosis which, at first glance, may be mistaken for reactive germinal centers [185, 187, 188]. The immunophenotype of the PDCs in these proliferations is similar to that of normal reactive PDCs; however, aberrant expression of lymphoid or myeloid markers has been reported [185, 187, 189]. A clonal relationship with similar chromosomal abnormalities to the associated myeloid neoplasm has been demonstrated by fluorescent in-situ hybridization or mutational analysis in some cases [186, 187]. The prognosis generally depends on the underlying myeloid neoplasm [186, 188].
Langerhans Cell Proliferations associated with Lymphoma
Langerhans cell neoplasia (histiocytosis or sarcoma) has been observed in association with other hematologic malignancies. In such cases, the Langerhans cell proliferation may share common genetic abnormalities with the associated malignancy suggesting a clonal relationship or transdifferentiation [190–193]. Small foci of Langerhans cell histiocytosis (LCH) may also represent a reactive, incidental finding in lymphoma and such aggregates have been shown to be non-clonal by HUMARA assay [194]. A recent study did not find BRAF V600E or MAP2K1 mutations in the incidental LCH component of seven cases analyzed (associated with classical Hodgkin lymphoma, mantle cell lymphoma and angioimmunoblastic T-cell lymphoma). The authors concluded that the presence of lymphoma-associated LCH is a benign process, and suggested that activation of the ERK pathway may occur due to the lymphoma or its interplay with the associated microenvironment [195].
Histiocytic and Dendritic Cell Neoplasms
Current classifications of histiocytic and dendritic cell disorders include the WHO classification of histiocytic and dendritic cell neoplasms, which includes neoplasms of both hematopoietic and mesenchymal origin [196, 197] and the revised classification from the Histiocyte Society, which divides the histiocytoses and neoplasms of the macrophage-dendritic cell lineages into five groups based on the clinical, radiological, pathological and molecular characteristics of the diseases [4]. Histiocytic and dendritic cell neoplasms are rare tumors which show morphologic and immunophenotypic features of the various dendritic cell and macrophage subsets. Although uncertainties regarding the cell of origin remain for certain neoplasms, recent transcriptomic analysis has provided some additional insights.
Histiocytic sarcoma shares morphologic and immunophenotypic features with mature tissue histiocytes. It is a diagnosis of exclusion, as the tumor is positive for non-specific histiocytic markers, and should be negative for diagnostic markers of other entities. Langerhans cell histiocytosis has similar morphologic and immunophenotypic features to the Langerhans cell, as it is positive for CD1a and langerin, and ultrastructurally contains Birbeck granules. Transcriptomic analysis has suggested closer similarity between the neoplastic cells of LCH and immature myeloid-dendritic cells than epidermal Langerhans cells, suggesting that LCH may arise from a more immature cell than the differentiated Langerhans cell from which it has been proposed to arise [198]. Interdigitating dendritic cell sarcoma is postulated to arise from the interdigitating dendritic cell, whereas follicular dendritic cell sarcoma and fibroblastic reticulum cell tumor are tumors of the mesenchymally-derived follicular dendritic and fibroblastic reticulum cells [197].
Blastic plasmacytoid dendritic cell neoplasm is probably derived from immediate precursors of plasmacytoid dendritic cells [185, 197]. It expresses CD4, CD56, CD123 and BDCA2(CD303) [185]. In contrast to the previous neoplasms, it is grouped with the myeloid neoplasms and acute leukemias and not the histiocytic and dendritic cell neoplasms in the WHO classification [199].
Conclusion
Accumulations of benign histiocytes and dendritic cells can occur in lymph nodes in response to diverse stimuli including foreign material, infection, autoimmunity, tumor and as a result of lysosomal storage disorders. Although they may present as lymphadenopathy, such proliferations are also encountered as an unexpected finding in lymph nodes excised for other purposes, such as cancer staging. Certain histiocytic and dendritic cell infiltrates have distinctive histopathological features, correct recognition of which should prompt appropriate ancillary tests or clinical correlation to identify an underlying etiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128(3):415–35. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R, et al. Mononuclear phagocytic system: new classification of macrophages, monocytes and of their cell line. Bull World Health Organ. 1972;47(5):651–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emile JF, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–81. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guilliams M, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guilliams M, van de Laar L. A Hitchhiker’s Guide to Myeloid Cell Subsets: Practical Implementation of a Novel Mononuclear Phagocyte Classification System. Front Immunol. 2015;6:406. doi: 10.3389/fimmu.2015.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Favara BE, et al. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29(3):157–66. doi: 10.1002/(sici)1096-911x(199709)29:3<157::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Haniffa M, Bigley V, Collin M. Human mononuclear phagocyte system reunited. Semin Cell Dev Biol. 2015;41:59–69. doi: 10.1016/j.semcdb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Collin M, Milne P. Langerhans cell origin and regulation. Curr Opin Hematol. 2016;23(1):28–35. doi: 10.1097/MOH.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella M, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5(8):919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 11.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15(8):471–85. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14(6):392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 13.Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Front Immunol. 2015;6:486. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krautler NJ, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150(1):194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol. 2015;15(6):350–61. doi: 10.1038/nri3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20(1):14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heesters BA, Myers RC, Carroll MC. Follicular dendritic cells: dynamic antigen libraries. Nat Rev Immunol. 2014;14(7):495–504. doi: 10.1038/nri3689. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251(1):160–76. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black MM, Kerpe S, Speer FD. Lymph Node Structure in Patients with Cancer of the Breast. The American Journal of Pathology. 1953;29(3):505–521. [PMC free article] [PubMed] [Google Scholar]
- 21.Berg JW. Sinus histiocytosis: a fallacious measure of host resistance to cancer. Cancer. 1956;9(5):935–9. doi: 10.1002/1097-0142(195609/10)9:5<935::aid-cncr2820090511>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg SG, et al. Sinus histiocytosis and mammary carcinoma. Study of 366 radical mastectomies and an historical review. Cancer. 1970;26(6):1177–85. doi: 10.1002/1097-0142(197012)26:6<1177::aid-cncr2820260602>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Gould E, et al. Signet ring cell sinus histiocytosis. A previously unrecognized histologic condition mimicking metastatic adenocarcinoma in lymph nodes. Am J Clin Pathol. 1989;92(4):509–12. doi: 10.1093/ajcp/92.4.509. [DOI] [PubMed] [Google Scholar]
- 24.Cappellari JO, Iskandar SS, Woodruff RD. Signet ring cell sinus histiocytosis. Am J Clin Pathol. 1990;94(6):800–1. doi: 10.1093/ajcp/94.6.800. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero-Medrano J, Delgado R, Albores-Saavedra J. Signet-ring sinus histiocytosis: a reactive disorder that mimics metastatic adenocarcinoma. Cancer. 1997;80(2):277–85. doi: 10.1002/(sici)1097-0142(19970715)80:2<277::aid-cncr16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Allen CE, et al. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50(6):1227–35. doi: 10.1002/pbc.21423. [DOI] [PubMed] [Google Scholar]
- 27.Meeths M, et al. Incidence and clinical presentation of primary hemophagocytic lymphohistiocytosis in Sweden. Pediatr Blood Cancer. 2015;62(2):346–352. doi: 10.1002/pbc.25308. [DOI] [PubMed] [Google Scholar]
- 28.Allen CE, McClain KL. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematology Am Soc Hematol Educ Program. 2015;2015:177–82. doi: 10.1182/asheducation-2015.1.177. [DOI] [PubMed] [Google Scholar]
- 29.Janka GE. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. 1983;140(3):221–30. doi: 10.1007/BF00443367. [DOI] [PubMed] [Google Scholar]
- 30.Riviere S, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med. 2014;127(11):1118–25. doi: 10.1016/j.amjmed.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, et al. A clinical analysis of 52 adult patients with hemophagocytic syndrome: the prognostic significance of the underlying diseases. Int J Hematol. 2001;74(2):209–13. doi: 10.1007/BF02982007. [DOI] [PubMed] [Google Scholar]
- 32.Henter JI, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 33.Janka GE, Lehmberg K. Hemophagocytic syndromes--an update. Blood Rev. 2014;28(4):135–42. doi: 10.1016/j.blre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Pachlopnik Schmid J, et al. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev. 2010;235(1):10–23. doi: 10.1111/j.0105-2896.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139(6):713–27. doi: 10.1309/AJCP4ZDKJ4ICOUAT. [DOI] [PubMed] [Google Scholar]
- 36.Stepp SE, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286(5446):1957–9. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 37.Feldmann J, et al. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115(4):461–73. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 38.zur Stadt U, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14(6):827–34. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 39.zur Stadt U, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18–2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85(4):482–92. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagel J, et al. Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5) Blood. 2012;119(25):6016–24. doi: 10.1182/blood-2011-12-398958. [DOI] [PubMed] [Google Scholar]
- 41.Meeths M, et al. Clinical presentation of Griscelli syndrome type 2 and spectrum of RAB27A mutations. Pediatr Blood Cancer. 2010;54(4):563–72. doi: 10.1002/pbc.22357. [DOI] [PubMed] [Google Scholar]
- 42.Nagle DL, et al. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nat Genet. 1996;14(3):307–11. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 43.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166(2):95–109. doi: 10.1007/s00431-006-0258-1. [DOI] [PubMed] [Google Scholar]
- 44.Enders A, et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108(1):81–7. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- 45.Jessen B, et al. The risk of hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type 2. Blood. 2013;121(15):2943–51. doi: 10.1182/blood-2012-10-463166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols KE, et al. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–99. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 47.Latour S, Aguilar C. XIAP deficiency syndrome in humans. Semin Cell Dev Biol. 2015;39:115–23. doi: 10.1016/j.semcdb.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Jordan MB, et al. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041–52. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos-Casals M, et al. Adult haemophagocytic syndrome. Lancet. 2014;383(9927):1503–16. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 50.Hsi ED, Schnitzer B. Reactive Lymphadenopathies. In: Jaffe ES, et al., editors. Hematopathology. Elsevier; Philadelphia, PA: 2017. pp. 153–77. [Google Scholar]
- 51.Sullivan JL, et al. Epstein-Barr virus-associated hemophagocytic syndrome: virological and immunopathological studies. Blood. 1985;65(5):1097–104. [PubMed] [Google Scholar]
- 52.Buckley PJ, O’Laughlin S, Komp DM. Histiocytes in familial and infection-induced/idiopathic hemophagocytic syndromes may exhibit phenotypic differences. Pediatr Pathol. 1992;12(1):51–66. doi: 10.3109/15513819209023280. [DOI] [PubMed] [Google Scholar]
- 53.Kogawa K, et al. Perforin expression in cytotoxic lymphocytes from patients with hemophagocytic lymphohistiocytosis and their family members. Blood. 2002;99(1):61–6. doi: 10.1182/blood.v99.1.61. [DOI] [PubMed] [Google Scholar]
- 54.Suga N, et al. Perforin defects of primary haemophagocytic lymphohistiocytosis in Japan. Br J Haematol. 2002;116(2):346–9. doi: 10.1046/j.1365-2141.2002.03266.x. [DOI] [PubMed] [Google Scholar]
- 55.Gupta A, Weitzman S, Abdelhaleem M. The role of hemophagocytosis in bone marrow aspirates in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;50(2):192–4. doi: 10.1002/pbc.21441. [DOI] [PubMed] [Google Scholar]
- 56.Listinsky CM. Common reactive erythrophagocytosis in axillary lymph nodes. Am J Clin Pathol. 1988;90(2):189–92. doi: 10.1093/ajcp/90.2.189. [DOI] [PubMed] [Google Scholar]
- 57.Fitzgerald PJ, Kinney TD. Intestinal Lipodystrophy (Whipple’s Disease) Am J Pathol. 1945;21(6):1069–89. [PMC free article] [PubMed] [Google Scholar]
- 58.Maizel H, Ruffin JM, Dobbins WO., 3rd Whipple’s disease: a review of 19 patients from one hospital and a review of the literature since 1950. Medicine (Baltimore) 1970;49(3):175–205. [PubMed] [Google Scholar]
- 59.Morgan AD. The first recorded case of Whipple’s disease? Gut. 1961;2:370–2. doi: 10.1136/gut.2.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Relman DA, et al. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med. 1992;327(5):293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- 61.Li W, et al. Genotyping reveals a wide heterogeneity of Tropheryma whipplei. Microbiology. 2008;154(Pt 2):521–7. doi: 10.1099/mic.0.2007/011668-0. [DOI] [PubMed] [Google Scholar]
- 62.Rollin DC, et al. Genotypic analysis of Tropheryma whipplei from patients with Whipple disease in the Americas. J Clin Pathol. 2017 doi: 10.1136/jclinpath-2017-204382. [DOI] [PubMed] [Google Scholar]
- 63.Puite RH, Tesluk H. Whipple’s disease. Am J Med. 1955;19(3):383–400. doi: 10.1016/0002-9343(55)90127-3. [DOI] [PubMed] [Google Scholar]
- 64.Marth T, et al. Tropheryma whipplei infection and Whipple’s disease. Lancet Infect Dis. 2016;16(3):e13–22. doi: 10.1016/S1473-3099(15)00537-X. [DOI] [PubMed] [Google Scholar]
- 65.Dolmans RA, et al. Clinical Manifestations, Treatment, and Diagnosis of Tropheryma whipplei Infections. Clin Microbiol Rev. 2017;30(2):529–555. doi: 10.1128/CMR.00033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Enzinger FM, Helwig EB. Whipple’s disease. Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin. 1963;336(3):238–269. [Google Scholar]
- 67.Fleming JL, Wiesner RH, Shorter RG. Whipple’s disease: clinical, biochemical, and histopathologic features and assessment of treatment in 29 patients. Mayo Clin Proc. 1988;63(6):539–51. doi: 10.1016/s0025-6196(12)64884-8. [DOI] [PubMed] [Google Scholar]
- 68.Lagier JC, Fenollar F, Raoult D. Acute infections caused by Tropheryma whipplei. Future Microbiol. 2017;12:247–254. doi: 10.2217/fmb-2017-0178. [DOI] [PubMed] [Google Scholar]
- 69.Keita AK, Raoult D, Fenollar F. Tropheryma whipplei as a commensal bacterium. Future Microbiol. 2013;8(1):57–71. doi: 10.2217/fmb.12.124. [DOI] [PubMed] [Google Scholar]
- 70.Martinetti M, et al. The HLA alleles DRB1*13 and DQB1*06 are associated to Whipple’s disease. Gastroenterology. 2009;136(7):2289–94. doi: 10.1053/j.gastro.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 71.Desnues B, et al. Whipple disease: intestinal infiltrating cells exhibit a transcriptional pattern of M2/alternatively activated macrophages. J Infect Dis. 2005;192(9):1642–6. doi: 10.1086/491745. [DOI] [PubMed] [Google Scholar]
- 72.Marth T, et al. Defects of monocyte interleukin 12 production and humoral immunity in Whipple’s disease. Gastroenterology. 1997;113(2):442–8. doi: 10.1053/gast.1997.v113.pm9247462. [DOI] [PubMed] [Google Scholar]
- 73.Moos V, et al. Impaired immune functions of monocytes and macrophages in Whipple’s disease. Gastroenterology. 2010;138(1):210–20. doi: 10.1053/j.gastro.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 74.Biagi F, et al. Cytokine genetic profile in Whipple’s disease. Eur J Clin Microbiol Infect Dis. 2012;31(11):3145–50. doi: 10.1007/s10096-012-1677-8. [DOI] [PubMed] [Google Scholar]
- 75.Marth T, et al. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple’s disease. Gastroenterology. 2002;123(5):1468–77. doi: 10.1053/gast.2002.36583. [DOI] [PubMed] [Google Scholar]
- 76.Schinnerling K, et al. Regulatory T cells in patients with Whipple’s disease. J Immunol. 2011;187(8):4061–7. doi: 10.4049/jimmunol.1101349. [DOI] [PubMed] [Google Scholar]
- 77.Moos V, et al. Reduced peripheral and mucosal Tropheryma whipplei-specific Th1 response in patients with Whipple’s disease. J Immunol. 2006;177(3):2015–22. doi: 10.4049/jimmunol.177.3.2015. [DOI] [PubMed] [Google Scholar]
- 78.Marth T. Tropheryma whipplei, Immunosuppression and Whipple’s Disease: From a Low-Pathogenic, Environmental Infectious Organism to a Rare, Multifaceted Inflammatory Complex. Dig Dis. 2015;33(2):190–9. doi: 10.1159/000369538. [DOI] [PubMed] [Google Scholar]
- 79.Ghigo E, et al. IL-16 promotes T. whipplei replication by inhibiting phagosome conversion and modulating macrophage activation. PLoS One. 2010;5(10):e13561. doi: 10.1371/journal.pone.0013561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desnues B, Raoult D, Mege JL. IL-16 is critical for Tropheryma whipplei replication in Whipple’s disease. J Immunol. 2005;175(7):4575–82. doi: 10.4049/jimmunol.175.7.4575. [DOI] [PubMed] [Google Scholar]
- 81.Alkan S, Beals TF, Schnitzer B. Primary diagnosis of whipple disease manifesting as lymphadenopathy: use of polymerase chain reaction for detection of Tropheryma whippelii. Am J Clin Pathol. 2001;116(6):898–904. doi: 10.1309/7678-E2DW-HFJ5-QYUJ. [DOI] [PubMed] [Google Scholar]
- 82.Van Bockstal M, et al. Whipple’s disease in granulomatous disguise: a challenging diagnosis with many histopathological pitfalls. Virchows Arch. 2017;470(4):465–468. doi: 10.1007/s00428-017-2084-4. [DOI] [PubMed] [Google Scholar]
- 83.Wilcox GM, et al. Periodic acid-Schiff-negative granulomatous lymphadenopathy in patients with Whipple’s disease. Localization of the Whipple bacillus to noncaseating granulomas by electron microscopy. Am J Med. 1987;83(1):165–70. doi: 10.1016/0002-9343(87)90514-6. [DOI] [PubMed] [Google Scholar]
- 84.Black-Schaffer B. The tinctoral demonstration of a glycoprotein in Whipple’s disease. Proc Soc Exp Biol Med. 1949;72(1):225–7. doi: 10.3181/00379727-72-17388. [DOI] [PubMed] [Google Scholar]
- 85.Spapen HD, et al. Electron microscopic detection of Whipple’s bacillus in sarcoidlike periodic acid-Schiff-negative granulomas. Dig Dis Sci. 1989;34(4):640–3. doi: 10.1007/BF01536345. [DOI] [PubMed] [Google Scholar]
- 86.Morningstar WA. Whipple’s disease. An example of the value of the electron microscope in diagnosis, follow-up, and correlation of a pathologic process. Hum Pathol. 1975;6(4):443–54. doi: 10.1016/s0046-8177(75)80062-1. [DOI] [PubMed] [Google Scholar]
- 87.Durand DV, et al. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Societe Nationale Francaise de Medecine Interne. Medicine (Baltimore) 1997;76(3):170–84. doi: 10.1097/00005792-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Feurle GE, et al. The immune reconstitution inflammatory syndrome in whipple disease: a cohort study. Ann Intern Med. 2010;153(11):710–7. doi: 10.7326/0003-4819-153-11-201012070-00004. [DOI] [PubMed] [Google Scholar]
- 89.Freemont A. The pathology of joint replacement and tissue engineering. Diagnostic Histopathology. 2012;18(4):169–176. [Google Scholar]
- 90.Basle MF, et al. Migration of metal and polyethylene particles from articular prostheses may generate lymphadenopathy with histiocytosis. J Biomed Mater Res. 1996;30(2):157–63. doi: 10.1002/(SICI)1097-4636(199602)30:2<157::AID-JBM4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 91.Benz EB, et al. Lymphadenopathy associated with total joint prostheses. A report of two cases and a review of the literature. J Bone Joint Surg Am. 1996;78(4):588–93. doi: 10.2106/00004623-199604000-00014. [DOI] [PubMed] [Google Scholar]
- 92.Catelas I, Wimmer MA, Utzschneider S. Polyethylene and metal wear particles: characteristics and biological effects. Semin Immunopathol. 2011;33(3):257–71. doi: 10.1007/s00281-011-0242-3. [DOI] [PubMed] [Google Scholar]
- 93.Gray MH, et al. Changes seen in lymph nodes draining the sites of large joint prostheses. Am J Surg Pathol. 1989;13(12):1050–6. doi: 10.1097/00000478-198912000-00007. [DOI] [PubMed] [Google Scholar]
- 94.O’Connell JX, Rosenberg AE. Histiocytic lymphadenitis associated with a large joint prosthesis. Am J Clin Pathol. 1993;99(3):314–6. doi: 10.1093/ajcp/99.3.314. [DOI] [PubMed] [Google Scholar]
- 95.Hicks DG, et al. Granular histiocytosis of pelvic lymph nodes following total hip arthroplasty. The presence of wear debris, cytokine production, and immunologically activated macrophages. J Bone Joint Surg Am. 1996;78(4):482–96. doi: 10.2106/00004623-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Albores-Saavedra J, et al. Sinus histiocytosis of pelvic lymph nodes after hip replacement. A histiocytic proliferation induced by cobalt-chromium and titanium. Am J Surg Pathol. 1994;18(1):83–90. doi: 10.1097/00000478-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 97.Bos I, et al. Comparative investigations of regional lymph nodes and pseudocapsules after implantation of joint endoprostheses. Pathol Res Pract. 1990;186(6):707–16. doi: 10.1016/S0344-0338(11)80260-8. [DOI] [PubMed] [Google Scholar]
- 98.Peoc’h M, et al. Foreign body histiocytosis reaction after hip replacement with concomitant metastatic adenocarcinoma in the same lymph node. Hum Pathol. 1998;29(1):95–8. doi: 10.1016/s0046-8177(98)90397-5. [DOI] [PubMed] [Google Scholar]
- 99.Willert HG, Ludwig J, Semlitsch M. Reaction of bone to methacrylate after hip arthroplasty: a long-term gross, light microscopic, and scanning electron microscopic study. J Bone Joint Surg Am. 1974;56(7):1368–82. [PubMed] [Google Scholar]
- 100.Bos I, Johannisson R. Foreign body reactions in lymph nodes of oncology patients with joint prostheses--light-, electron microscopic and immunohistological investigations. Pathol Res Pract. 2004;200(3):189–96. doi: 10.1016/j.prp.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 101.Hausner RJ, Schoen FJ, Pierson KK. Foreign-body reaction to silicone gel in axillary lymph nodes after an augmentation mammaplasty. Plast Reconstr Surg. 1978;62(3):381–4. doi: 10.1097/00006534-197809000-00007. [DOI] [PubMed] [Google Scholar]
- 102.Christie AJ, Weinberger KA, Dietrich M. Silicone lymphadenopathy and synovitis. Complications of silicone elastomer finger joint prostheses. Jama. 1977;237(14):1463–4. [PubMed] [Google Scholar]
- 103.Omakobia E, et al. Silicone lymphadenopathy: an unexpected cause of neck lumps. J Laryngol Otol. 2012;126(9):970–3. doi: 10.1017/S0022215112001089. [DOI] [PubMed] [Google Scholar]
- 104.Collado-Mesa F, et al. Contralateral intramammary silicone lymphadenitis in a patient with an intact standard dual-lumen breast implant in the opposite reconstructed breast. J Radiol Case Rep. 2013;7(11):24–31. doi: 10.3941/jrcr.v7i11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Travis WD, Balogh K, Abraham JL. Silicone granulomas: report of three cases and review of the literature. Hum Pathol. 1985;16(1):19–27. doi: 10.1016/s0046-8177(85)80209-4. [DOI] [PubMed] [Google Scholar]
- 106.Truong LD, et al. Silicone lymphadenopathy associated with augmentation mammaplasty. Morphologic features of nine cases. Am J Surg Pathol. 1988;12(6):484–91. doi: 10.1097/00000478-198806000-00009. [DOI] [PubMed] [Google Scholar]
- 107.Katzin WE, et al. Pathology of lymph nodes from patients with breast implants: a histologic and spectroscopic evaluation. Am J Surg Pathol. 2005;29(4):506–11. doi: 10.1097/01.pas.0000155145.60670.e4. [DOI] [PubMed] [Google Scholar]
- 108.Kuo ACM. Poly(dimethylsiloxane) In: Mark JE, editor. Polymer Data Handbook. Oxford University Press; New York: 1999. pp. 411–32. [Google Scholar]
- 109.Corrin B. Silicone lymphadenopathy. J Clin Pathol. 1982;35(8):901–2. doi: 10.1136/jcp.35.8.901-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogers LA, et al. Silicone lymphadenopathy in a long distance runner: complication of a silastic prosthesis. Hum Pathol. 1988;19(10):1237–9. doi: 10.1016/s0046-8177(88)80158-8. [DOI] [PubMed] [Google Scholar]
- 111.Hardt NS, et al. Macrophage-silicone interactions in women with breast prostheses. Curr Top Microbiol Immunol. 1996;210:245–52. doi: 10.1007/978-3-642-85226-8_24. [DOI] [PubMed] [Google Scholar]
- 112.Peoc’h M, et al. Silicone lymphadenopathy mimicking a lymphoma in a patient with a metatarsophalangeal joint prosthesis. J Clin Pathol. 2000;53(7):549–51. doi: 10.1136/jcp.53.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gil T, et al. Contralateral internal mammary silicone lymphadenopathy imitates breast cancer metastasis. Ann Plast Surg. 2009;63(1):39–41. doi: 10.1097/SAP.0b013e318188d092. [DOI] [PubMed] [Google Scholar]
- 114.D’Hulst L, et al. False-Positive Axillary Lymph Nodes Due to Silicone Adenitis on (18)F-FDG PET/CT in an Oncological Setting. J Thorac Oncol. 2016;11(6):e73–5. doi: 10.1016/j.jtho.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 115.Dogan S, Barnes L, Cruz-Vetrano WP. Crystal-storing histiocytosis: report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012;6(1):111–20. doi: 10.1007/s12105-011-0326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kapadia SB, et al. Crystal-storing histiocytosis associated with lymphoplasmacytic neoplasms. Report of three cases mimicking adult rhabdomyoma. Am J Surg Pathol. 1993;17(5):461–7. doi: 10.1097/00000478-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Harada M, et al. Crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma mimicking Weber-Christian disease: immunohistochemical, ultrastructural, and gene-rearrangement studies. Hum Pathol. 1996;27(1):84–7. doi: 10.1016/s0046-8177(96)90143-4. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto T, et al. Crystal-storing histiocytosis and crystalline tissue deposition in multiple myeloma. Arch Pathol Lab Med. 1991;115(4):351–4. [PubMed] [Google Scholar]
- 119.de Alba Campomanes AG, et al. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea. 2009;28(9):1081–4. doi: 10.1097/ICO.0b013e318199f73b. [DOI] [PubMed] [Google Scholar]
- 120.Bosman C, et al. Solitary crystal-storing histiocytosis of the tongue in a patient with rheumatoid arthritis and polyclonal hypergammaglobulinemia. Arch Pathol Lab Med. 1998;122(10):920–4. [PubMed] [Google Scholar]
- 121.Joo M, et al. Localized gastric crystal-storing histiocytosis. Histopathology. 2007;51(1):116–9. doi: 10.1111/j.1365-2559.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 122.Kaminsky IA, et al. Central nervous system crystal-storing histiocytosis: neuroimaging, neuropathology, and literature review. AJNR Am J Neuroradiol. 2011;32(2):E26–8. doi: 10.3174/ajnr.A1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sukpanichnant S, et al. Clofazimine-induced crystal-storing histiocytosis producing chronic abdominal pain in a leprosy patient. Am J Surg Pathol. 2000;24(1):129–35. doi: 10.1097/00000478-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 124.Gebrail F, et al. Crystalline histiocytosis in hereditary cystinosis. Arch Pathol Lab Med. 2002;126(9):1135. doi: 10.5858/2002-126-1135-CHIHC. [DOI] [PubMed] [Google Scholar]
- 125.Lewis JT, et al. Crystal-storing histiocytosis due to massive accumulation of charcot-leyden crystals: a unique association producing colonic polyposis in a 78-year-old woman with eosinophilic colitis. Am J Surg Pathol. 2007;31(3):481–5. doi: 10.1097/01.pas.0000213420.46127.9c. [DOI] [PubMed] [Google Scholar]
- 126.Jones D, et al. Crystal-storing histiocytosis: a disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol. 1999;30(12):1441–8. doi: 10.1016/s0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 127.Lebeau A, et al. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: molecular analysis of a disorder with rapid clinical course and review of the literature. Blood. 2002;100(5):1817–27. [PubMed] [Google Scholar]
- 128.Kanagal-Shamanna R, et al. Crystal-storing histiocytosis: a clinicopathological study of 13 cases. Histopathology. 2016;68(4):482–91. doi: 10.1111/his.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Takahashi K, et al. Multiple myeloma, IgA kappa type, accompanying crystal-storing histiocytosis and amyloidosis. Acta Pathol Jpn. 1987;37(1):141–54. doi: 10.1111/j.1440-1827.1987.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 130.Terashima K, et al. Kappa-type light chain crystal storage histiocytosis. Acta Pathol Jpn. 1978;28(1):111–38. doi: 10.1111/j.1440-1827.1978.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 131.Conalty ML, V, Barry C, Jina A. The antileprosy agent B.663 (Clofazimine) and the reticuloendothelial system. Int J Lepr Other Mycobact Dis. 1971;39(2):479–92. [PubMed] [Google Scholar]
- 132.Prasad ML, et al. Pulmonary immunocytoma with massive crystal storing histiocytosis: a case report with review of literature. Am J Surg Pathol. 1998;22(9):1148–53. doi: 10.1097/00000478-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 133.Garcia JF, et al. Crystal-storing histiocytosis and immunocytoma associated with multifocal fibrosclerosis. Histopathology. 1998;33(5):459–64. doi: 10.1046/j.1365-2559.1998.00531.x. [DOI] [PubMed] [Google Scholar]
- 134.McKay Bounford K, Gissen P. Genetic and laboratory diagnostic approach in Niemann Pick disease type C. J Neurol. 2014;261(Suppl 2):S569–75. doi: 10.1007/s00415-014-7386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schuchman EH, Wasserstein MP. Types A and B Niemann-Pick disease. Best Pract Res Clin Endocrinol Metab. 2015;29(2):237–47. doi: 10.1016/j.beem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 136.Chen M, Wang J. Gaucher disease: review of the literature. Arch Pathol Lab Med. 2008;132(5):851–3. doi: 10.5858/2008-132-851-GDROTL. [DOI] [PubMed] [Google Scholar]
- 137.Thomas AS, Mehta A, Hughes DA. Gaucher disease: haematological presentations and complications. Br J Haematol. 2014;165(4):427–40. doi: 10.1111/bjh.12804. [DOI] [PubMed] [Google Scholar]
- 138.Weisberger J, Emmons F, Gorczyca W. Cytochemical diagnosis of Gaucher’s disease by iron stain. Br J Haematol. 2004;124(6):696. doi: 10.1046/j.1365-2141.2003.04695.x. [DOI] [PubMed] [Google Scholar]
- 139.Williams GT, Williams WJ. Granulomatous inflammation--a review. J Clin Pathol. 1983;36(7):723–33. doi: 10.1136/jcp.36.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shah KK, Pritt BS, Alexander MP. Histopathologic review of granulomatous inflammation. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases. 2017;7:1–12. doi: 10.1016/j.jctube.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Timmermans WM, et al. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin Transl Immunology. 2016;5(12):e118. doi: 10.1038/cti.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cinetto F, Agostini C. Advances in understanding the immunopathology of sarcoidosis and implications on therapy. Expert Rev Clin Immunol. 2016;12(9):973–88. doi: 10.1080/1744666X.2016.1181541. [DOI] [PubMed] [Google Scholar]
- 143.McClean CM, Tobin DM. Macrophage form, function, and phenotype in mycobacterial infection: lessons from tuberculosis and other diseases. Pathog Dis. 2016;74(7) doi: 10.1093/femspd/ftw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wessendorf TE, Bonella F, Costabel U. Diagnosis of Sarcoidosis. Clin Rev Allergy Immunol. 2015;49(1):54–62. doi: 10.1007/s12016-015-8475-x. [DOI] [PubMed] [Google Scholar]
- 145.Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24(3):150–61. doi: 10.1053/j.semdp.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 146.Sheffield EA. Pathology of sarcoidosis. Clin Chest Med. 1997;18(4):741–54. doi: 10.1016/s0272-5231(05)70416-0. [DOI] [PubMed] [Google Scholar]
- 147.Cain H, Kraus B. Asteroid bodies: derivatives of the cytosphere. An electron microscopic contribution to the pathology of the cytocentre. Virchows Arch B Cell Pathol. 1977;26(2):119–32. [PubMed] [Google Scholar]
- 148.Ioachim HL, Medeiros LJ. Ioachim’s Lymph Node Pathology. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. Sarcoidosis Lymphadenopathy; pp. 203–12. [Google Scholar]
- 149.Tudway AJ. Yellow bodies in superficial and deep lymph nodes. J Clin Pathol. 1979;32(1):52–5. doi: 10.1136/jcp.32.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Winters R, Kida M, Cooper K. Hamazaki-Wesenberg bodies in two patients with no history of sarcoidosis. J Clin Pathol. 2007;60(10):1169–71. doi: 10.1136/jcp.2006.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Geboes K, et al. The cellular composition of granulomas in mesenteric lymph nodes from patients with Crohn’s disease. Virchows Arch A Pathol Anat Histopathol. 1986;409(5):679–92. doi: 10.1007/BF00713433. [DOI] [PubMed] [Google Scholar]
- 152.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13(3):147–56. doi: 10.1016/0305-7372(86)90002-2. [DOI] [PubMed] [Google Scholar]
- 153.Norbis L, et al. Challenges and perspectives in the diagnosis of extrapulmonary tuberculosis. Expert Rev Anti Infect Ther. 2014;12(5):633–47. doi: 10.1586/14787210.2014.899900. [DOI] [PubMed] [Google Scholar]
- 154.Ioachim HL, Medeiros LJ. Ioachim’s Lymph Node Pathology. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. Mycobacterium Tuberculosis Lymphadenitis; pp. 130–36. [Google Scholar]
- 155.Fukunaga H, et al. Sensitivity of acid-fast staining for Mycobacterium tuberculosis in formalin-fixed tissue. Am J Respir Crit Care Med. 2002;166(7):994–7. doi: 10.1164/rccm.2111028. [DOI] [PubMed] [Google Scholar]
- 156.Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–80. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Binford CH. Histoplasmosis: tissue reactions and morphologic variations of the fungus. Am J Clin Pathol. 1955;25(1):25–36. doi: 10.1093/ajcp/25.1.25. [DOI] [PubMed] [Google Scholar]
- 158.Winship T. Pathologic changes in so-called cat-scratch fever; review of findings in lymph node of 29 patients and cutaneous lesions of 2 patients. Am J Clin Pathol. 1953;23(10):1012–8. doi: 10.1093/ajcp/23.10.1012. [DOI] [PubMed] [Google Scholar]
- 159.Naji AF, Carbonell F, Barker HJ. Cat scratch disease. A report of three new cases, review of the literature, and classification of the pathologic changes in the lymph nodes during various stages of the disease. Am J Clin Pathol. 1962;38:513–21. doi: 10.1093/ajcp/38.5.513. [DOI] [PubMed] [Google Scholar]
- 160.Miller-Catchpole R, et al. Cat scratch disease. Identification of bacteria in seven cases of lymphadenitis. Am J Surg Pathol. 1986;10(4):276–81. [PubMed] [Google Scholar]
- 161.Wear DJ, et al. Cat scratch disease: a bacterial infection. Science. 1983;221(4618):1403–5. doi: 10.1126/science.6612349. [DOI] [PubMed] [Google Scholar]
- 162.Cheuk W, et al. Confirmation of diagnosis of cat scratch disease by immunohistochemistry. Am J Surg Pathol. 2006;30(2):274–5. doi: 10.1097/01.pas.0000190325.35204.ee. [DOI] [PubMed] [Google Scholar]
- 163.Scott MA, et al. Cat scratch disease: detection of Bartonella henselae DNA in archival biopsies from patients with clinically, serologically, and histologically defined disease. Am J Pathol. 1996;149(6):2161–7. [PMC free article] [PubMed] [Google Scholar]
- 164.Hadfield TL, Lamy Y, Wear DJ. Demonstration of Chlamydia trachomatis in inguinal lymphadenitis of lymphogranuloma venereum: a light microscopy, electron microscopy and polymerase chain reaction study. Mod Pathol. 1995;8(9):924–9. [PubMed] [Google Scholar]
- 165.Sutinen S, Syrjala H. Histopathology of human lymph node tularemia caused by Francisella tularensis var palaearctica. Arch Pathol Lab Med. 1986;110(1):42–6. [PubMed] [Google Scholar]
- 166.Moller O. Dermatopathic lymphadenitis. Acta Pathol Microbiol Scand. 1951;28(4):352–65. [PubMed] [Google Scholar]
- 167.Hurwitt E. Dermatopathic Lymphadenitis. Focal Granulomatous Lymphadenitis Associated with Chronic Generalized Skin Disorders. Journal of Investigative Dermatology. 1942;5(4):197–204. [Google Scholar]
- 168.Wise F. Lymphadenosis cutis universalis, associated with generalized erythrodermia and atrophy of the skin. J Cut Dis. 1917;35:669–704. [Google Scholar]
- 169.Cooper RA, Dawson PJ, Rambo ON. Dermatopathic lymphadenopathy a clinicopathologic analysis of lymph node biopsy over a fifteen-year period. Calif Med. 1967;106(3):170–5. [PMC free article] [PubMed] [Google Scholar]
- 170.Steffen C. Frederic Woringer: Pautrier-Woringer disease (lipomelanotic reticulosis/dermatopathic lymphadenitis) Am J Dermatopathol. 2004;26(6):499–503. doi: 10.1097/00000372-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 171.Scheffer E, Meijer CJ, Van Vloten WA. Dermatopathic lymphadenopathy and lymph node involvement in mycosis fungoides. Cancer. 1980;45(1):137–48. doi: 10.1002/1097-0142(19800101)45:1<137::aid-cncr2820450124>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 172.Schnyder UW, Schirren CG. So-called “lipomelanotic reticulosis” of pautrier-woringer. AMA Archives of Dermatology and Syphilology. 1954;70(2):155–165. doi: 10.1001/archderm.1954.01540200015002. [DOI] [PubMed] [Google Scholar]
- 173.Burke JS, Colby TV. Dermatopathic lymphadenopathy. Comparison of cases associated and unassociated with mycosis fungoides. Am J Surg Pathol. 1981;5(4):343–52. [PubMed] [Google Scholar]
- 174.Gould E, et al. Dermatopathic lymphadenitis. The spectrum and significance of its morphologic features. Arch Pathol Lab Med. 1988;112(11):1145–50. [PubMed] [Google Scholar]
- 175.van der Oord JJ, et al. The paracortical area in dermatopathic lymphadenitis and other reactive conditions of the lymph node. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(3):289–99. doi: 10.1007/BF02889871. [DOI] [PubMed] [Google Scholar]
- 176.Shamoto M, et al. Do epidermal Langerhans cells, migrating from skin lesions, induce the paracortical hyperplasia of dermatopathic lymphadenopathy? Pathol Int. 1996;46(5):348–54. doi: 10.1111/j.1440-1827.1996.tb03620.x. [DOI] [PubMed] [Google Scholar]
- 177.Ioachim HL, Medeiros LJ. Ioachim’s Lymph Node Pathology. Lippincott Williams & Wilkins; 2009. Dermatopathic Lymphadenitis; pp. 223–226. [Google Scholar]
- 178.Herrera GA. Light microscopic, S-100 immunostaining, and ultrastructural analysis of dermatopathic lymphadenopathy, with and without associated mycosis fungoides. Am J Clin Pathol. 1987;87(2):187–95. doi: 10.1093/ajcp/87.2.187. [DOI] [PubMed] [Google Scholar]
- 179.Burke JS, Sheibani K, Rappaport H. Dermatopathic lymphadenopathy. An immunophenotypic comparison of cases associated and unassociated with mycosis fungoides. Am J Pathol. 1986;123(2):256–63. [PMC free article] [PubMed] [Google Scholar]
- 180.Assaf C, et al. Early TCR-beta and TCR-gamma PCR detection of T-cell clonality indicates minimal tumor disease in lymph nodes of cutaneous T-cell lymphoma: diagnostic and prognostic implications. Blood. 2005;105(2):503–10. doi: 10.1182/blood-2004-06-2220. [DOI] [PubMed] [Google Scholar]
- 181.Fraser-Andrews EA, et al. Molecular staging of lymph nodes from 60 patients with mycosis fungoides and Sezary syndrome: correlation with histopathology and outcome suggests prognostic relevance in mycosis fungoides. Br J Dermatol. 2006;155(4):756–62. doi: 10.1111/j.1365-2133.2006.07428.x. [DOI] [PubMed] [Google Scholar]
- 182.Dargent JL, et al. Plasmacytoid dendritic cells in Kimura disease. Am J Dermatopathol. 2009;31(8):854–6. doi: 10.1097/DAD.0b013e3181a772c6. [DOI] [PubMed] [Google Scholar]
- 183.Rollins-Raval MA, et al. The number and growth pattern of plasmacytoid dendritic cells vary in different types of reactive lymph nodes: an immunohistochemical study. Hum Pathol. 2013;44(6):1003–10. doi: 10.1016/j.humpath.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 184.Facchetti F, et al. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003;443(6):703–17. doi: 10.1007/s00428-003-0918-8. [DOI] [PubMed] [Google Scholar]
- 185.Facchetti F, et al. Neoplasms derived from plasmacytoid dendritic cells. Mod Pathol. 2016;29(2):98–111. doi: 10.1038/modpathol.2015.145. [DOI] [PubMed] [Google Scholar]
- 186.Vermi W, et al. Nodal and extranodal tumor-forming accumulation of plasmacytoid monocytes/interferon-producing cells associated with myeloid disorders. Am J Surg Pathol. 2004;28(5):585–95. doi: 10.1097/00000478-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 187.Tzankov A, et al. Plasmacytoid dendritic cell proliferations and neoplasms involving the bone marrow: Summary of the workshop cases submitted to the 18th Meeting of the European Association for Haematopathology (EAHP) organized by the European Bone Marrow Working Group, Basel 2016. Ann Hematol. 2017;96(5):765–777. doi: 10.1007/s00277-017-2947-4. [DOI] [PubMed] [Google Scholar]
- 188.Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16(6):392–404. doi: 10.1097/PAP.0b013e3181bb6bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Facchetti F, et al. Leukemia-associated lymph node infiltrates of plasmacytoid monocytes (so-called plasmacytoid T-cells). Evidence for two distinct histological and immunophenotypical patterns. Am J Surg Pathol. 1990;14(2):101–12. doi: 10.1097/00000478-199002000-00001. [DOI] [PubMed] [Google Scholar]
- 190.Yokokawa Y, et al. Unique clonal relationship between T-cell acute lymphoblastic leukemia and subsequent Langerhans cell histiocytosis with TCR rearrangement and NOTCH1 mutation. Genes Chromosomes Cancer. 2015;54(7):409–17. doi: 10.1002/gcc.22252. [DOI] [PubMed] [Google Scholar]
- 191.West DS, et al. Clonally related follicular lymphomas and Langerhans cell neoplasms: expanding the spectrum of transdifferentiation. Am J Surg Pathol. 2013;37(7):978–86. doi: 10.1097/PAS.0b013e318283099f. [DOI] [PubMed] [Google Scholar]
- 192.Feldman AL, et al. Clonal relationship between precursor T-lymphoblastic leukaemia/lymphoma and Langerhans-cell histiocytosis. Lancet Oncol. 2005;6(6):435–7. doi: 10.1016/S1470-2045(05)70211-4. [DOI] [PubMed] [Google Scholar]
- 193.Furmanczyk PS, et al. Langerhans cell sarcoma in a patient with hairy cell leukemia: common clonal origin indicated by identical immunoglobulin gene rearrangements. J Cutan Pathol. 2012;39(6):644–50. doi: 10.1111/j.1600-0560.2012.01873.x. [DOI] [PubMed] [Google Scholar]
- 194.Christie LJ, et al. Lesions resembling Langerhans cell histiocytosis in association with other lymphoproliferative disorders: a reactive or neoplastic phenomenon? Hum Pathol. 2006;37(1):32–9. doi: 10.1016/j.humpath.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 195.Pina-Oviedo S, et al. Langerhans cell histiocytosis associated with lymphoma: an incidental finding that is not associated with BRAF or MAP2K1 mutations. Mod Pathol. 2017 doi: 10.1038/modpathol.2016.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. IARC WHO Classification of Tumours. 4. Lyon: World Health Organization; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; p. 441. [Google Scholar]
- 198.Allen CE, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184(8):4557–67. doi: 10.4049/jimmunol.0902336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Arber DA, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 200.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 201.Ohadi M, et al. Localization of a gene for familial hemophagocytic lymphohistiocytosis at chromosome 9q21.3–22 by homozygosity mapping. Am J Hum Genet. 1999;64(1):165–71. doi: 10.1086/302187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ioachim HL, Medeiros LJ. Ioachim’s Lymph Node Pathology. Lippincott Williams & Wilkins; Philadelphia, PA: 2009. Cryptococcus Lymphadenopathy; pp. 147–49. [Google Scholar]
- 203.Srinivasan R, et al. Cryptococcal lymphadenitis diagnosed by fine needle aspiration cytology: a review of 15 cases. Acta Cytol. 2010;54(1):1–4. doi: 10.1159/000324958. [DOI] [PubMed] [Google Scholar]
- 204.Stoner BP, Cohen SE. Lymphogranuloma Venereum 2015: Clinical Presentation, Diagnosis, and Treatment. Clin Infect Dis. 2015;61(Suppl 8):S865–73. doi: 10.1093/cid/civ756. [DOI] [PubMed] [Google Scholar]