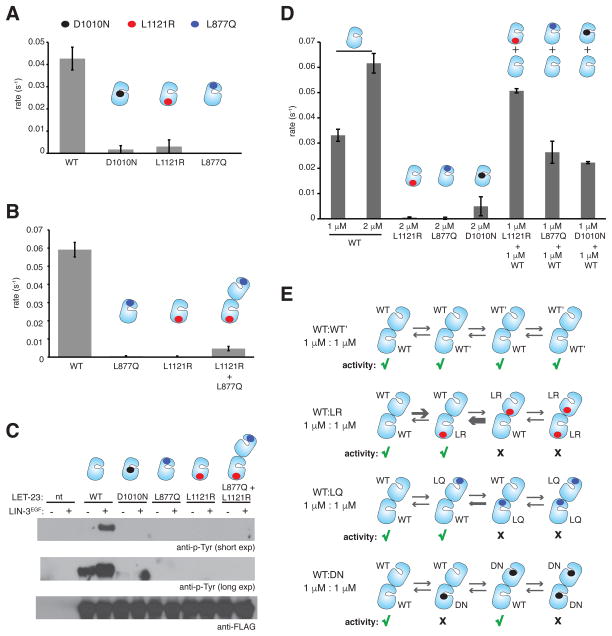
Figure 5. Role of the asymmetric dimer interface in activation of the LET-23 kinase domain.
(A) Specific activity of the wild-type and mutant LET-23 kinase constructs at 1 μM, linked to vesicles containing 5 mole percent DOGS-NTA-Ni. (B) Specific activity measurements for the wild-type, L877Q, and L1121R constructs alone collected at 2 μM protein concentrations. In the L877Q/L1121R condition, each protein was present in the reaction mixture at 1 μM (2 μM total concentration). Data were collected on vesicles containing 5 mole percent DOGS-NTA-Ni. (C) Western blot analysis of LIN-3EGF-induced phosphorylation of full-length wild type of mutant LET-23 constructs upon transient transfection in COS-7 cells and immunoprecipitation with the anti-FLAG antibody. LIN-3EGF corresponds to the purified EGF domain from LIN-3, as previously described (Freed et al., 2015). (D) Specific activity of the wild-type and mutant LET-23 kinase constructs linked to vesicles containing 5 mole percent DOGS-NTA-Ni. (E) Hypothesized EGFR-like asymmetric dimer pairings between the wild type and mutant LET-23 kinases mixed at equimolar concentrations. Green check marks indicate activity, black crosses indicate no activity. Specific activity data are represented as mean ± S.D.