Fig. 7.
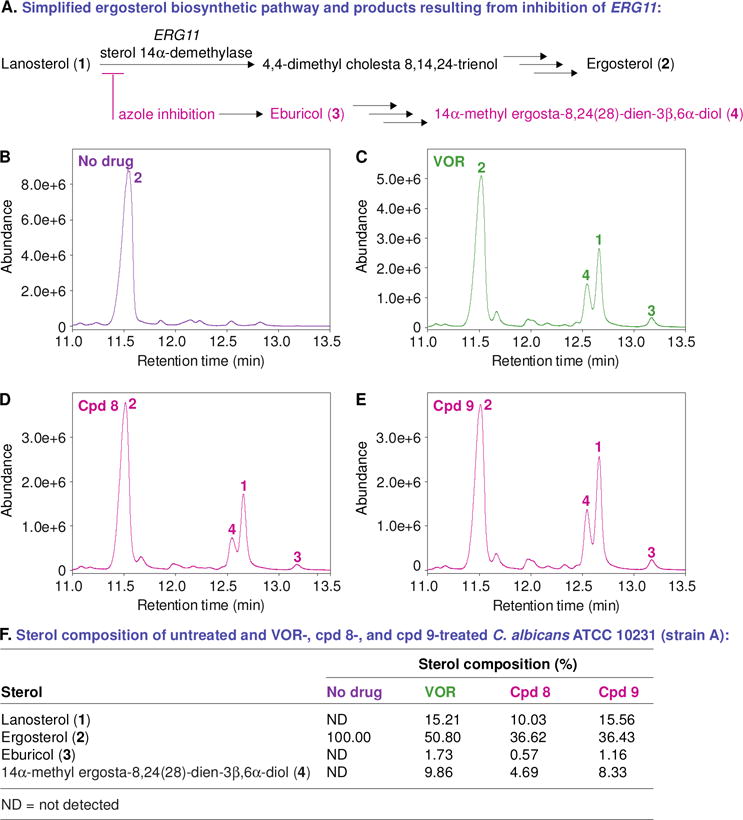
A. A simplified ergosterol biosynthetic pathway and products resulting from inhibition of ERG11. B–E. GC-MS chromatograms of the sterols extracted from untreated and antifungal-treated C. albicans ATCC 10231 (strain A). The fungal strain treated with DMSO (no drug control, panel B), VOR at 0.12 μg/mL (panel C), compound 8 at 0.48 μg/mL (panel D), and compound 9 at 0.975 μg/mL (panel E). The peaks in these chromatograms are for lanosterol (1), ergosterol (2), eburicol (3), 14α-methyl ergosta-8,24(28)-dien-3β,6α-diol (4). F. A table summarizing the percentage of each sterol from panels B–E.
