Abstract
Background
Kawasaki disease (KD) is the most common acquired heart disease in children of the developed world, and triggers progressive coronary artery lesions (CAL) in 30% of cases if left untreated. Despite standard anti-inflammatory treatment for KD, CAL (dilation or aneurysm) still occurs in 5–10% of children, increasing their risk for fatal coronary artery complications. CAL is mediated by enhanced matrix metalloproteinase activity and elastin breakdown induced by the inflammatory process in the coronary artery wall. Doxycycline is an effective inhibitor of matrix metalloproteinases, and has been shown to reduce elastin breakdown and CAL in a mouse model of KD, but has not been evaluated in patients.
Objective
We aim to evaluate the efficacy of doxycycline in the prevention of CAL in children during the acute phase of KD.
Design
This is a phase II prospective, randomized, double-blinded, clinical trial in two steps. In Step 1, any child older than 1 month with the diagnosis of KD will be included. Children with KD will be included in Step 2 if they develop coronary artery dilation (z-score ≥ 2.5) within 20 days from the onset of fever. Study subjects in Step 2 will be randomized to receive a 3-week course of doxycycline or placebo.
Evaluation
The efficacy of a 3-week doxycycline course during the acute phase of KD will be evaluated by measuring the decline in coronary artery z-scores from baseline with doxycycline treatment compared to placebo.
Clinical trial registration
This study was registered on clinicaltrials.gov (NCT01917721).
Introduction
Kawasaki disease (KD) is a multisystem vasculitis that affects the coronary arteries of young children (1). It is the leading cause of acquired heart disease in children in the developed world (2). Even though the systemic inflammation in KD will resolve without any treatment, the associated vasculitis may result in irreversible damage of the coronary arteries, which can lead to the major sequel of KD: coronary artery dilation or aneurysm (4, 5). In the absence of treatment, about 25–30% of children with KD will develop irreversible coronary artery dilation or small aneurysm (6). Even following current standard therapeutic guidelines with standard intravenous gamma globulin (IVIG) treatment, about 30% of children may have transient coronary artery dilation, and 5–10% of children may still develops permanent coronary artery lesions including aneurysms (7). Dilated coronary arteries and coronary aneurysms increase the short-term risk of thrombosis, myocardial infarction and may even lead to death. Permanent coronary arterial wall damage can also contribute to long-term complications, such as coronary artery stenosis and ischemic heart disease (4, 5, 8, 9).
Coronary artery lesions (CAL), meaning dilation or aneurysm, are the result of inflammation and secondary destruction of the coronary vessel wall (10). In KD, the immune system, triggered by a so far unknown extrinsic factor, induces inflammation of the coronary arteries, macrophage and lymphocyte activation (11, 12), and degradation of certain extracellular matrix elements, such as elastin, collagen and laminin (13). These extracellular matrix elements are targets of matrix metalloproteinases (MMPs), which have increased activity in the coronary arteries and blood during the acute phase of KD (14, 15, 16). The degree of MMP-9 activity in the coronary arteries correlates with the development of CAL in a mouse model of KD (17), and there is evidence that circulating pro-MMP-9 and MMP-9 levels (16, 18, 19) are increased during the acute phase of KD in children, correlating with the degree of inflammation and the occurrence of CAL. In summary, MMPs may trigger the degradation of elastin and other matrix elements, and could play an important role in the development of CAL in the acute phase of KD.
Among the known inhibitors of MMPs, doxycycline has been studied extensively due to its long established use and safety (20). Doxycycline has been shown to block MMP activity (21), decrease elastin breakdown in aortic aneurysms (22), and inhibit progressive vessel dilation by inhibiting proteolysis (23). In vivo administration of doxycycline to mice with a model of KD reduces degradation of elastin, and prevents coronary artery dilation (24). However, the efficacy of doxycycline in preventing CAL has not yet been evaluated in children with KD. We propose a prospective, randomized, placebo-controlled, double-blind phase II clinical trial to assess the efficacy of a 3-week doxycycline treatment in the prevention of progressive dilation of the coronary arteries in children during the acute phase of KD.
Rationale and objective
There is no specific therapy available to prevent the progression of coronary artery dilation or aneurysm formation in children with KD. Doxycycline is presently not used in children with KD, in part due to the paucity of appropriate clinical studies of efficacy. Based on the in vitro and in vivo evidence, MMP inhibition by doxycycline may reduce the degradation of elastin and prevent progression of CAL during the acute inflammatory phase of KD. The primary objective of the study is to evaluate the efficacy of doxycycline in preventing the progressive dilation of coronary arteries during the acute phase of KD in children.
Methods
Study Design
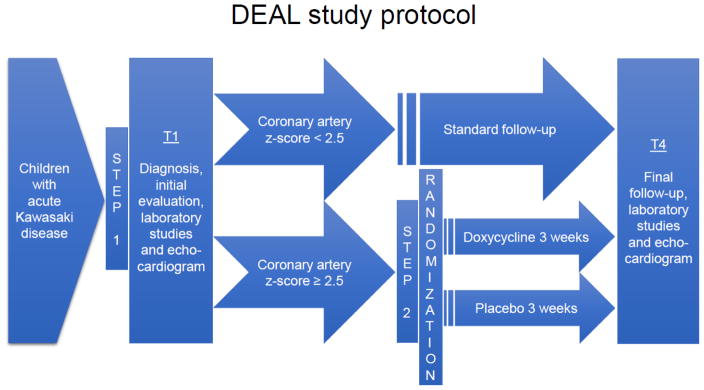
This is a Phase II prospective randomized placebo-controlled double-blinded clinical trial conducted in children ages 1 month to 18 years. There will be two steps of enrollment in the trial: all children with KD will be eligible to be enrolled in Step 1, and children with KD and coronary artery dilation will be eligible to be enrolled in Step 2. Only study subjects in Step 2 (i.e. children with KD and coronary artery dilation) will receive the proposed intervention: doxycycline or placebo (Figure 1).
Figure 1.
Treatment protocol for the DEAL trial.
Study Population
The study population will consist of children, ages 1 month to 18 years, hospitalized with the diagnosis of acute KD. Regardless of gender and ethnic background, every child will be included in Step 1 of the study if they meet the inclusion criterion for the diagnosis of KD. All study subjects will have an echocardiogram as part of the standard KD diagnostic protocol, as per international guidelines (8). Study subjects will be stratified depending on their echocardiogram results: study subjects lacking clearly enlarged coronary arteries (coronary artery diameter z-scores < 2.5) will participate only in Step 1, and study subjects with the presence of coronary artery dilation or aneurysm (i.e., coronary artery diameter z-score ≥ 2.5 during the first 3 weeks of the illness from the onset of fever) will participate in Step 1 and be eligible to participate in Step 2 part of the study. Study subjects in Step 2 (children with KD and coronary artery dilation) will be assigned to one of the two treatment arms: doxycycline treatment or placebo treatment (Figure 1). The assignment will follow a predetermined randomization key. Since gender may influence the outcome, we will randomize patients stratified by gender. The clinicians, caregivers and collaborators except pharmacists will be blinded to the treatment. The complete list of inclusion and exclusion criteria is listed in Table 1. Based on our previous experience, the vast majority of patients will be between 6 months and 5 years of age. Since KD is more prevalent in children of Asian descent, we will expect to see more children enrolled with Asian ethnicity.
Table 1.
Inclusion and exclusion criteria for Step 1 and Step 2
| Step 1 | Step 2 | |
|---|---|---|
| Inclusion criteria | Ages 1 month to 18 years | As in Step 1 |
| Diagnosis of Kawasaki disease: 5 days of fever and 4 out of 5 symptoms (conjunctival injection, palmar or solar edema, cervical lymphadenopathy, lingual or labial erythema, maculopapular rash) | ||
| Diagnosis and hospitalization within 3 weeks of fever onset | ||
| Informed consent (and assent) | ||
| Coronary artery dilation with a z-score ≥ 2.5 | ||
| Exclusion criteria | Denial of consent/assent | As in Step 1 |
| Fever onset more than 3 weeks prior to diagnosis and hospitalization | ||
| Tetracycline or sulfite allergy or prior treatment with tetracyclines (< 8 years of age) | ||
| Kidney failure (creatinine level above the upper limit of normal for age) | ||
| Liver failure (AST, ALT, bilirubin or GGT level elevated 5x above the upper limit of normal for age) |
Study interventions
Study subjects in Step 2 (i.e. children with KD and coronary artery dilation) will be randomized into two arms: treatment arm and placebo arm. The treatment arm will receive doxycycline treatment as an intervention, the placebo arm will receive placebo. All other diagnostic and therapeutic procedures are intended to be the same for the two arms. The randomization assignment will follow a predetermined block randomization key. The randomization key will be only available to the study pharmacist, all other participants will be blinded. To prevent potential randomization imbalances arising from gender distribution or age in the randomized arms, we will use stratified randomization method by gender and age separating study subjects below and above 1 year of age. In addition, the final statistical analysis will consider and address possible imbalances in the randomized arms given the rather small number of subjects participating in this pilot study.
Intervention: doxycycline treatment
Study subjects in the drug treatment arm will be administered doxycycline for three weeks. The doxycycline treatment will be initiated as soon as the patient fulfills the inclusion criteria: documented coronary artery dilation detected by echocardiogram. The initiation of study drug therefore will almost always fall into the first few days of hospitalization for KD, because CAL occur during the acute phase of KD, most likely within the first 2 weeks from the onset of fever. We determined the 3 week duration of doxycycline treatment based on the timing of acute inflammation in KD and the safety profile of doxycycline. Subjects will receive the previously established standard anti-infective pediatric doxycycline treatment dose of 4.4 mg/kg/day divided twice a day (20). Study subjects will have a physical examination and standard laboratory testing as part of their hospitalization for KD. Patients with a known allergy to sulfa drugs or tetracycline or a history of renal dysfunction (indicated by elevated creatinine level) will be excluded from the study. As >45% of patients with acute KD have transient elevation in liver function tests, we will exclude only those with bilirubin, transaminase or GGT over five times the upper limit of normative age appropriate values. Doxycycline will be administered in solution format. Parents and study subjects will be educated about the potential side effects of the study drugs (doxycycline). In case of any adverse reaction or side effect related to the study drugs (doxycycline), the study drug will be discontinued, the study subject will be evaluated in a timely manner and potential adverse effects will be addressed and treated by appropriate medical professionals. If any evidence of liver or kidney dysfunction (evidenced by elevated liver enzymes or serum creatinine as mentioned above) or an allergic reaction emerges, study drug will be stopped immediately.
Intervention: placebo administration
In the control arm of the study, placebo will be administered. The timing, amount and duration of placebo administration will be exactly the same as the active study drug. The placebo will be prepared by an established pharmaceutical company and will contain sugar solution (syrup). A dedicated Research Pharmacist will prepare the placebo similar to the doxycycline. In case of an allergic reaction to the placebo, the study drug will be discontinued immediately and the study subject evaluated by medical professionals.
Data collection
The following data will be collected from all participating study subjects: demographics (age, gender, ethnicity) and clinical measurements including height, weight, body surface area, and vital signs on admission, at the time of echocardiogram, maximum temperature measured by tympanic digital thermometer, duration of fever; duration of hospital stay; medications administered; standard laboratory data (comprehensive metabolic panel, complete blood count, C-reactive protein, erythrocyte sedimentation rate); echocardiogram measurements (diameter of the coronary arteries, left ventricle dimensions). Coronary artery diameters will be measured at the proximal segment of the right coronary artery and the left anterior descending coronary artery according to established standards (26). Z-scores will be calculated from the coronary artery measurements based on the body surface area according to established normative standards (26). In addition to the above, serum samples will be collected from study subjects in order to evaluate potential biomarkers of CAL. We will use a tiered approach that includes a global proteomic screen, evaluation of previously established markers, and confirmation of specific candidate proteins. Among the candidate proteins we will include acute phase inflammatory markers, interleukins, elastin degradation products, matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases and markers associated with cardiac and coronary artery inflammation. Some of these markers are elevated in the serum of children during the acute phase of KD, while others are associated with coronary artery damage in animal models. We will assess the correlation of serum concentration of candidate proteins with the degree of coronary artery dilation and the response to doxycycline. Data will be collected at 4 time points (#1: at the time of diagnosis; #2: 7–14 days after diagnosis; #3: 3–6 weeks after diagnosis; and #4: 8–16 weeks after diagnosis, i.e. in the convalescent phase) aligned to the standard diagnostic and treatment protocol for children with KD.
Primary and important secondary outcome measures
The primary endpoint of the study is the absolute change in coronary artery diameter z-score (standard deviation scoring measure based to established standards using body surface area) from time point 1 (acute phase, day of diagnosis of CAL) to time point 4 (convalescent phase, 8–16 weeks after the diagnosis of KD) in response to doxycycline treatment compared to placebo. We will follow measurements of a single coronary artery in each study subject (proximal segment of the right coronary artery OR left anterior descending coronary artery) depending on the largest initial dilation.
Important secondary endpoints are: 1) absolute change in coronary artery diameter z-score from time point 1 (acute phase) to time point 2 (early treatment phase, 1–2 weeks after diagnosis, within 7–10 days of study drug treatment) in response to doxycycline treatment compared to placebo; 2) relative change of coronary artery diameter z-score from time point 1 to time points 2 and 4 in response to doxycycline treatment; 3) ratio of subjects with normalized coronary artery diameter z-score by time point 4 (convalescent phase) in response to doxycycline treatment.
Drug safety and ethical aspects
The Food and Drug Administration (FDA) warns clinicians that doxycycline may cause tooth discoloration if used under the age of 8 years. Study subjects in this trial will receive only a 3-week course of doxycycline. This will ensure minimal cumulative dental side effect. We have conducted a systematic review of the literature to investigate the potential side effects of doxycycline treatment in children. An independent retrospective study including 140 children treated with doxycycline showed that the degree of tooth discoloration was not universal and it correlated with the length of treatment (30). In children with a single course of no more than 3 weeks of doxycycline treatment, none of the children had notable discoloration (30). Only prolonged (more than 5 weeks) or repetitive (twice or more) administration of doxycycline resulted in noticeable discoloration. After presenting a comprehensive review to the FDA, an Investigational New Drug (IND #118690) approval for doxycycline in KD was awarded to our investigators, allowing a single course (less than 5 weeks) of treatment in children of any age. In view of the above evidence, we will use a single course of treatment for 3 weeks, and will exclude subjects under the age of 8 years, who received prior tetracycline treatment.
Due to the potential side effect of dental discoloration, we will continue to collect dental data from study subjects even after completing study participation and treatment with doxycycline. Development of permanent teeth starts at 5 months of age and the crown development of incisors and premolars conclude by 6 years of age, but some of these teeth will be visible only by 13–14 years of age. Since all patients with KD follow up at the KD clinic as part of a standard protocol until they are 18 years old (patients with CAL follow up annually, patients without CAL follow up every 3–5 years with a final visit at 18 years), all study subjects will be followed at Kapiolani Medical Center for Women & Children long enough that their permanent teeth can be evaluated for dental discoloration. We will inform parents in the consent about the possibility of tooth discoloration, and will obtain data from their annual routine dental appointments. In addition, dental discoloration of every subject will be assessed by a dentist using a dental discoloration assessment card and established scoring system (29, 30). Dental discoloration will be recorded for all study subjects and results will be reported to all regulatory bodies.
This research study has been reviewed and approved by the Western Institutional Review Board (IRB protocol # 20131197). Written informed consent will be obtained from parents or legal guardians prior to study participation, and written assent will be obtained from children when appropriate. The study monitoring will strictly follow FDA/IND and IRB guidelines. This study has been registered on clinicaltrials.gov (NCT01917721) and the record has been updated in accordance with clinicaltrials.gov guidelines.
A Data Safety Monitoring Board (DSMB) will be formed by members not included in this clinical trial to oversee the conduct of the clinical trial. In addition to the DSMB, a trained monitor from Hawai‘i Pacific Health Research Institute will conduct routine monitoring of this study to ensure compliance with all regulatory requirements and accuracy of study data. The study monitoring will strictly follow FDA/IND and IRB guidelines.
Statistical Design and Power
We have conducted a preliminary study enrolling 10 subjects in Step 2, randomizing them to doxycycline (n=5) and placebo (n=5) treatment arms. We have evaluated the coronary artery diameter in these 10 children and detected a trend suggesting reduction of coronary artery dilation in the doxycycline group compared to placebo. Based on our preliminary results, accounting for the primary endpoint of absolute change in coronary artery diameter z-score from time point 1 (acute phase, day of diagnosis of CAL) to time point 4, a power calculation shows that we will need 17 study subjects per group (total of 34 subjects) to achieve 80% power to detect an effect size of 1.0 z-score (based on the absolute change in average z-scores) using a two-sided two sample t-test. These initial 10 study subjects used for the power calculation will be excluded from the prospective trial and the final analysis.
Statistical analysis
Descriptive statistics will be used to summarize demographic and clinical characteristics of study subjects. To explore the effect of randomization, first, we will compare characteristics between treatment arms using Chi-squared tests or Fisher’s exact test for categorical variables and two-sample t-tests or Mann-Whitney test for continuous variables. Selection bias will be prevented by the prospective nature of the trial and the allowance of study participation regardless of any other criteria except for the diagnosis of KD. Allocation bias will be prevented by the double-blinded randomization of the trial and predetermined stratified block randomization key. Potential imbalances in randomization despite the stratified approach will be accounted for at the final analysis.
Analysis will be implemented based on intent-to-treat (ITT) principle. For primary objective, the absolute diameter and relative change in diameter will be compared prior to and after doxycycline treatment, using two-sample t test or Wilcoxon signed ranks test.
If any characteristics are significantly imbalanced, we will perform analysis of covariance to adjust for the imbalance in randomization. Additional covariates that describe demographic and health characteristics and other covariates such as medication will be included if they have a bivariate association with the outcome (p-value ≤ 0.10). The assumptions of linear model will be tested. For example, normality will be explored using graphical (e.g., Q-Q plot, histogram) and statistical (e.g., Kolmogorov-Smirnov test) methods. If normality is violated, we will try to transform using box-cox transformation or use a generalized linear model with appropriate link (e.g. log). In addition, to address the effect of doxycycline over time, a longitudinal analysis will be implemented using a growth curve model which allows us to have different intercepts (i.e., baseline z-score) as well as linear (i.e., linear rate of z-score change over time) and quadratic aspects of change (i.e., non-linear rate of z-score change).
The results of this pivotal phase II pivotal study will provide critical preliminary information to power a larger multi-center clinical trial if necessary.
Preliminary results
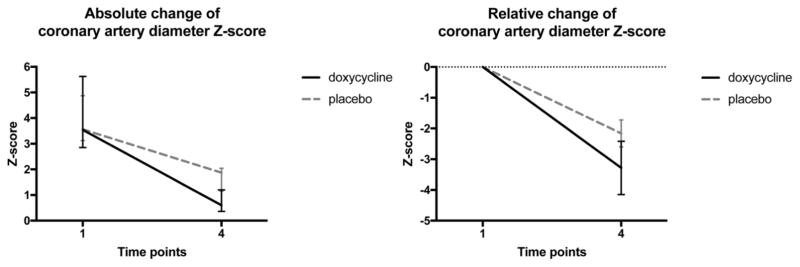
A pilot study obtained for power analysis included 10 study subjects randomized to the doxycycline arm (n=5) and the placebo arm (n=5). In our preliminary study, doxycycline was efficacious in preventing the progression of CAL (Figure 2), and may have contributed to decrease in CAL as evaluated by echocardiogram (Figure 3). This pilot trial served for our power calculation guiding the enrollment goals of this Phase II clinical trial.
Figure 2.
Coronary artery changes after doxycycline treatment compared to placebo Absolute and relative changes in coronary artery diameter z-score after doxycycline treatment compared to placebo during the acute phase of Kawasaki disease (KD). Ten patients were enrolled in a preliminary study and randomized to doxycycline (n=5) or placebo (n=5) treatment arms. From the acute phase of KD (T1) to the convalescent phase (T4), coronary artery diameter z-scores decreased more dramatically in the doxycycline arm compared to the placebo arm (with a relative change of z-score −2.55 vs. −1.57). This study served for our power calculation and sample size prediction.
Figure 3.
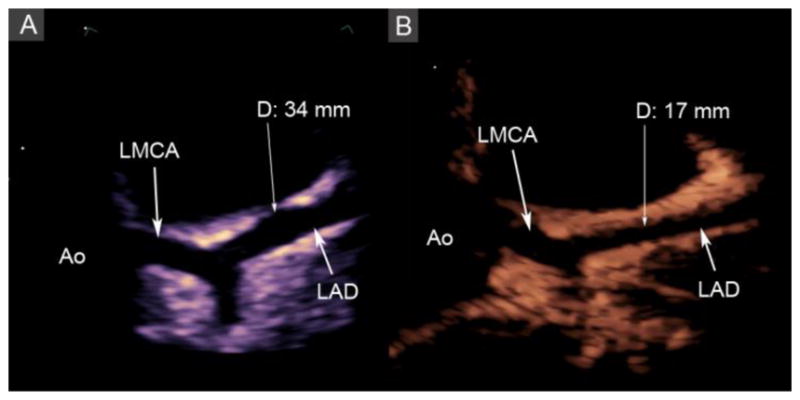
Echocardiograms of the left anterior descending coronary artery (LAD) during the acute and convalescent phases of Kawasaki disease (KD). A: Diffuse dilation of the LAD (arrow) up to 34 mm in diameter during the acute phase of KD. B: Resolution of the diffuse dilation (diameter 17 mm) during the convalescent phase after a 3-week treatment course of doxycycline, Ao: aorta, LMCA: left main coronary artery.
Discussion
If KD did not cause CAL, it would be just another self-limited febrile illness in children. The problem is that there is no therapy available to prevent the progression of coronary artery dilation and aneurysm formation in children with KD. There is no specific test that can detect KD and besides echocardiography, there are no laboratory tests that would indicate potential coronary artery damage in KD. Although IVIG improves the acute inflammation in most children with KD, it does not offer a therapeutic solution when CAL develops despite the established treatment protocol (7). There is insufficient evidence to guide the use of other therapies in patients with CAL, such as systemic steroids, infliximab and cyclosporine (31). Doxycycline is presently not used in children with KD due to the paucity of appropriate clinical studies of efficacy. Due to the lack of a specific therapy, patients can suffer from progressive vasculopathy of damaged coronary arteries at any age (5), which often leads to early invasive coronary interventions, or sometimes even coronary bypass surgery (9). There is a dearth of treatment options for patients with KD and CAL, and it is difficult to measure the efficacy of such treatment due to the lack of a biomarker reflecting CAL.
We are proposing a novel interventional concept not previously evaluated in children: implementing a treatment aimed to prevent CAL. We predict that doxycycline can mitigate MMP-mediated coronary elastin breakdown and improve coronary outcome in children during the acute phase of KD. The current pilot study could improve the treatment of KD and reduce the number of children who develop CAL due to KD. Since doxycycline is a strong inhibitor of MMPs, especially MMP-9, which may be responsible for the destruction of elastin in the coronary artery adventitia, and doxycycline has been shown efficacious in an animal model of KD to reduce CAL (25), it is an attractive candidate for clinical evaluation in children. If doxycycline proves to be efficacious in preventing the progression of CAL, this study will provide a new application for doxycycline as a novel therapeutic option for children with KD and CAL. Practitioners may finally have a therapeutic option for those KD patients, who develop CAL despite the standard treatment.
Our results could change the concept and treatment of Kawasaki disease. A multi-center study could be derived from our findings, or might not even be necessary. If such a study, or the results of this pivotal trial, compellingly confirms the efficacy of doxycycline in the treatment of children with KD and CAL, and validates biomarkers of CAL, one can anticipate widespread use of doxycycline for the prevention of MMP mediated vascular matrix destruction, and CAL in KD.
Acknowledgments
This study was supported by the Ingeborg v.F.McKee Fund of the Hawai‘i Community Foundation.
Statistical analysis by Eunjung Lim was supported in part by the grant U54MD007584 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawasaki T, Kosaka F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MCLS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 2.Uehara R, Belay E. Epidemiology of Kawasaki Disease in Asia, Europe and the United States. J Epidemiol. 2012;22:79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holman RC, Christensen KY, Belay ED, Steiner CA, Effler PV, Miyamura J, Forbes S, Schonberger LB, Melish ME. Racial/ethnic differences in the incidence of Kawasaki syndrome among children in Hawai’i. Hawai‘i Med J. 2010;69:194–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long Term consequences of Kawasaki Disease: a 10–21 year follow-up study of 594 patients. Circulation. 1996;93:1379–85. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 5.Burns JC, Shike H, Gordon JB, Malhotra A, Schoenwetter M, Kawasaki T. Sequelae of Kawasaki disease in adolescents and young adults. J Am Coll Cardiol. 1996;28:253–257. doi: 10.1016/0735-1097(96)00099-x. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, Shulman ST, Wiggins JW, Hicks RV, Fulton DR, Lewis AB, Leung DYM, Colton T, Rosen FS, Melish ME. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 7.Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, Atz AM, Li JS, Takahashi M, Baker AL, Colan SD, Mitchell PD, Klein GL, Sundel RP. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Eng J Med. 2007;15:356–375. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–1733. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 9.Suda K, Iemura M, Nishiono H, Teramachi Y, Koteda Y, Kishimoto S, Kudo Y, Itoh S, Ishii H, Ueno T, Tashiro T, Nobuyoshi M, Kato H, Matsuishi T. Long term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single institution experience. Circulation. 2011;123:1836–42. doi: 10.1161/CIRCULATIONAHA.110.978213. [DOI] [PubMed] [Google Scholar]
- 10.Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease. II. Distribution and incidence of the vascular lesions. Jpn Circ J. 1979;43:741–748. doi: 10.1253/jcj.43.741. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, Kato H, Yabuta K. Peripheral blood monocyte/macrophage and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988;48:247–251. doi: 10.1016/0090-1229(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 12.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 13.Sakata K, Hamaoka K, Ozawa S, Niboshi A, Yahata T, Fujii M, Hamaoka A, Toiyama K, Nishida M, Itoi T. Matrix metalloproteinase-9 in vascular lesions and endothelial regulation in Kawasaki disease. Circ J. 2010;74:1670–1675. doi: 10.1253/circj.cj-09-0980. [DOI] [PubMed] [Google Scholar]
- 14.Senzaki H, Masutani S, Kobayashi J, Kobayashi T, Nakano H, Nagasaka H, Sasaki N, Asano H, Kyo S, Yokote Y. Circulating matrix metalloproteinases and their inhibitors in patients with Kawasaki disease. Circulation. 2001;104:860–863. doi: 10.1161/hc3301.095286. [DOI] [PubMed] [Google Scholar]
- 15.Lau AC, Duong TT, Ito S, Yeung RS. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arth Rheum. 2008;58:854–863. doi: 10.1002/art.23225. [DOI] [PubMed] [Google Scholar]
- 16.Gavin PJ, Crawford SE, Shulman ST, Garcia FL, Rowley AH. Systemic arterial expression of matrix metalloproteinases 2 and 9 in acute Kawasaki disease. Arterioscler Thromb Vasc Biol. 2003;23:576–81. doi: 10.1161/01.ATV.0000065385.47152.FD. [DOI] [PubMed] [Google Scholar]
- 17.Lau AC, Rosenberg H, Duong TT, McCrindle BW, Yeung RSM. Elastolytic matrix metalloproteinases and coronary outcome in children with Kawasaki disease. Pediatr Res. 2007;61:710–715. doi: 10.1203/pdr.0b013e318053418b. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki T, Bossone E, Sawaki D, Janosi RA, Erbel R, Eagle K, Nagai R. Biomarkers of aortic diseases. Am Heart J. 2013;165:15–25. doi: 10.1016/j.ahj.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J Am Coll Cardiol. 2010;56:1535–1641. doi: 10.1016/j.jacc.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 20.Chua PK, Melish ME, Yu Q, Yanagihara R, Yamamoto KS, Nerurkar VR. Elevated levels of matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 during the acute phase of Kawasaki disease. Clin Diagn Lab Immunol. 2003;10:308–414. doi: 10.1128/CDLI.10.2.308-314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyle JR, McDermott E, Crowther M, Wills AD, Bell PR, Thompson MM. Doxycycline inhi bits elastin degradation and reduces metalloproteinase activity in a model of aneurysmal disease. J Vasc Surg. 1998;27:354–361. doi: 10.1016/s0741-5214(98)70367-2. [DOI] [PubMed] [Google Scholar]
- 22.Lindeman JH, Abdul-Hussein H, van Bockel JH, Wolterbeek R, Kleemann R. Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation. 2009;119:2209–2216. doi: 10.1161/CIRCULATIONAHA.108.806505. [DOI] [PubMed] [Google Scholar]
- 23.Abdul-Hussein H, Hanemaaijer R, Verheijen JH, van Bockel JH, Geelkerken RH, Lindeman JH. Doxycycline therapy for abdominal aneurysm: improved proteolytic balance through reduced neutrophil content. J Vasc Surg. 2009;49:741–749. doi: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 24.Lau AC, Duong TT, Ito S, Wilson GJ, Yeung RS. Inhibition of matrix metalloproteinase-9 activity improves coronary artery outcome in an animal model of Kawasaki disease. Clin Exp Immunol. 2009;157:300–309. doi: 10.1111/j.1365-2249.2009.03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratincsak A, Reddy VD, Purohit PJ, Tremoulet AH, Molkara DP, Frazer JR, Dyar D, Bush RA, Sim JY, Sang N, Burns JC, Melish MA. Coronary artery dilation in acute Kawasaki disease and acute illnesses associated with fever. Pediatr Infect Dis J. 2012;31:924–926. doi: 10.1097/INF.0b013e31826252b3. [DOI] [PubMed] [Google Scholar]
- 26.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–258. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 27.Pharmacokinetic, pharmacodynamics and toxicology data about doxycycline. Pfizer Inc; 1967. [Google Scholar]
- 28.Forti G, Benincori C. Doxycycline and the teeth. Lancet. 1969;1:782. doi: 10.1016/s0140-6736(69)91787-5. [DOI] [PubMed] [Google Scholar]
- 29.Grossman ER, Walchek A, Friedman H. Tetracycline and permanent teeth: the relation between does and tooth color. Pediatrics. 1971;47:567–570. [PubMed] [Google Scholar]
- 30.Product information: Vibranycin®, doxycycline oral capsules (hyclate), syrup (calcium), suspension (monohydrate) Pfizer Laboratories; New York, NY: 2003. [Google Scholar]
- 31.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E on behalf of the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]