Figure 2.
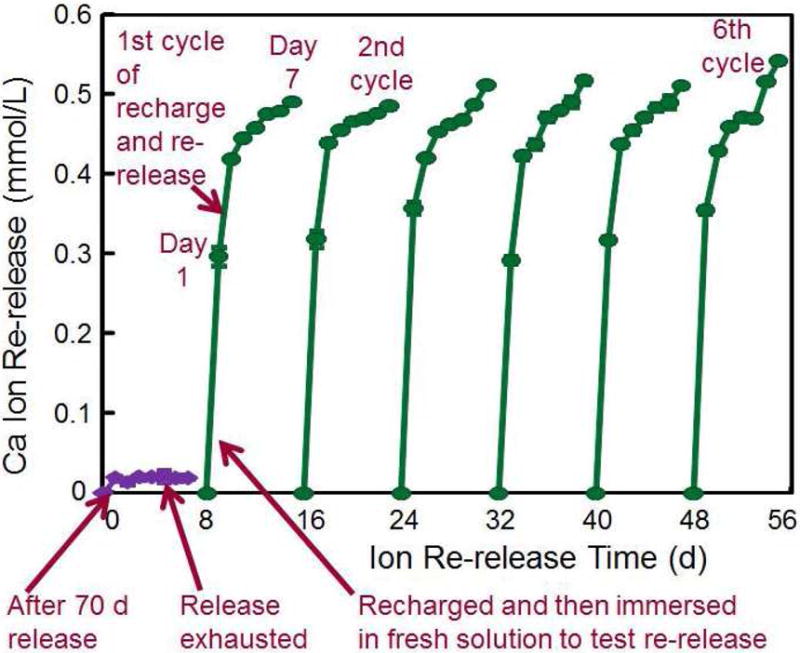
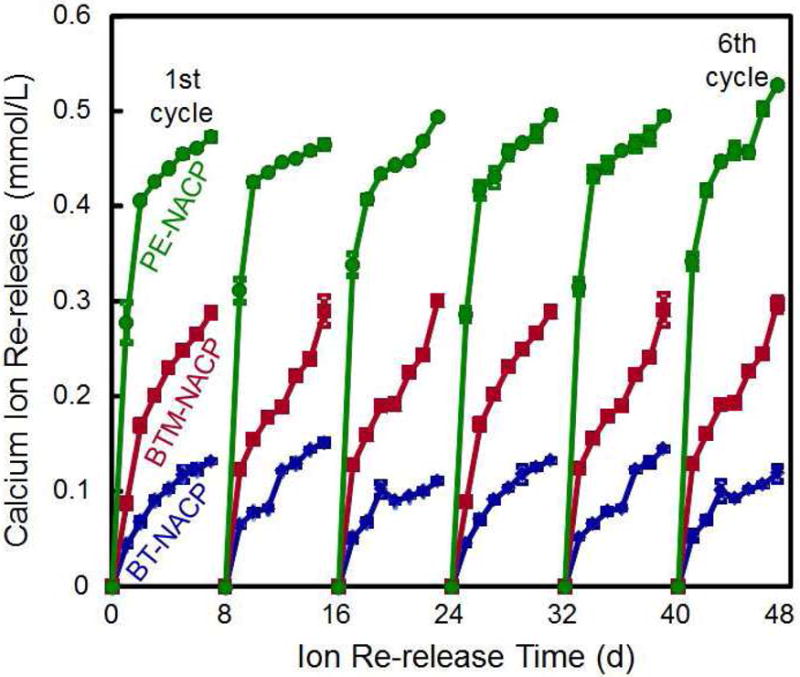
Rechargeable NACP composite for long-term Ca and P ion release. (A) NACP composite was immersed in a pH 4 solution for 70 d to exhaust the ion release (lower left arrow). Then the specimens were immersed in a new pH 4 solution to confirm that the ion release was exhausted (lower middle arrow). Specimens were recharged in a recharge solution, then tested for ion re-release for 7 d (third arrow at the bottom of A). This constituted the first recharge/re-release cycle. This process was repeated for 6 cycles. (B) Three NACP nanocomposites with six cycles of recharge/re-release, showing no decrease in ion release with increasing recharge cycles (p > 0.1).
Figure 2A: From Zhang L, Weir MD, Chow LC, et al. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater 2016;32(2):288; with permission.
Figure 2B: From Zhang L, Weir MD, Chow LC, et al. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater 2016;32(2):289; with permission.
