Abstract Abstract
Rhodactina is a small sequestrate genus in Boletaceae with two described species, R. himalayensis and R. incarnata. Phylogenetic analyses of a three-gene dataset including atp6, tef1 and rpb2 of Rhodactina species along with selected Boletaceae species showed that all Rhodactina species formed a monophyletic clade, sister to the genera Spongiforma and Borofutus in subfamily Leccinoideae with high support. All of the taxa in the clade have a similar chemical reaction in which basidiospores turn purplish, purplish red to violet or violet grey when in contact with potassium hydroxide. The molecular analyses also showed that all Rhodactina specimens collected from Ubon Ratchathani province, northeastern Thailand, belong to a new species. Morphologically, the new species is different from others by having a markedly prominent hilar appendage and a terminal hilum on its basidiospores. Thus, the new species, Rhodactina rostratispora, is introduced with detailed macroscopic and microscopic descriptions and illustrations.
Keywords: atp6, Boletales, Diversity, Leccinoideae, Phylogeny, Taxonomy
Introduction
The genus Rhodactina Pegler & T.W.K. Young was first described in 1989 with R. himalayensis Pegler & T.W.K. Young, from northwestern India, as the type species. Typical characters of the genus are a whitish to pinkish puffball like basidiomata lacking both stipe and columella, violet brown to purple brown or pale pink to red hymenophore when mature, combined with purplish to purplish red, dextrinoid basidiospores with longitudinal ridges, lack of both clamp connections and cystidia. The genus was originally classified based on morphological characters in the family Gautieriaceae Zeller as the spore ornamentation was originally viewed as similar to the genera Gautieria Vittad and Austrogautieria E.L. Stewart & Trappe (Pegler and Young 1989). In 2006, the second species, R. incarnata Zhu L. Yang, Trappe & Lumyong was described and the known distribution of R. himalayensis was extended to Chiang Mai Province, northern Thailand. Based on the phylogenetic analyses of atp6 sequences, the genus was moved to the family Boletaceae Chevall (Yang et al. 2006). However, the phylogenetic affinities of Rhodactina within the Boletaceae remained unclear because of very limited taxon sampling. So, at present, there are only two described Rhodactina species, R. himalayensis and R. incarnata (http://www.indexfungorum.org/Names/Names.asp), both of which have been reported to occur in northern Thailand (Chandrasrikul et al. 2011).
Boletaceae diversity seems to be high in Thailand (Chandrasrikul et al. 2011), with several new species described in the last five years (Choeyklin et al. 2012, Halling et al. 2014, Neves et al. 2012, Raspé et al. 2016). During this survey of Boletaceae diversity in Thailand, several Rhodactina collections were made and their morphology and phylogenetic relationships were studied. Phylogenetic analyses were based on three genes: atp6, tef1 and rpb2, which have previously been justified as useful for phylogenetic analyses of Boletales (Kretzer and Bruns 1999, Binder and Hibbett 2006, Hosen et al. 2013, Li et al. 2014, Smith et al. 2015, Orihara et al. 2016, Raspé et al. 2016, Wu et al. 2016). Both morphology and phylogenetic analyses confirmed that all newly collected specimens belong to a new species in the genus Rhodactina. Thus, the third species of Rhodactina, found in Thailand, is described and its phylogenetic affinities are presented in this study.
Materials and method
Specimens collecting
The new Rhodactina specimens were collected and photographed from community forests in Trakan Phuet Phon district, Ubon Ratchathani province, northeastern Thailand, in the rainy season during 2015–2017. The specimens were wrapped using aluminium foil or kept in plastic boxes until return to the laboratory and described within 24 h. The specimens were dried in an electric drier at 45–50 °C. The examined specimens are deposited in the herbaria CMUB and BR (both listed in Index Herbariorum; Thiers, continuously updated).
Morphological studies
The macroscopic description was based on detailed field notes and photos of basidiomata. Colour codes followed Kornerup and Wanscher (1978). Macrochemical reactions (colour reactions) of peridium, hymenophore and microscopic structures were determined using 5 % (w/v) aqueous potassium hydroxide, 28–30 % ammonium hydroxide or Melzer’s reagent. Microscopic structures were observed from dried specimens, rehydrated in 5% KOH or 1 % ammoniacal Congo red. For each collection, a minimum of 50 basidiospores and 20 basidia were randomly selected and measured at 1000× with a calibrated ocular micrometer using an Olympus CX31 microscope. Spore dimensions include ornamentation. The notation ‘(n/m/p)’ represents the number of basidiospores n measured from m basidiomata of p collections. Dimensions of microscopic structures are presented in the following format: (a–)b–c–d (–e), in which c represents the average, b the 5th percentile, d the 95th percentile and extreme values a and e are shown in parentheses. Q, the length/width ratio, is presented in the same format. Sections of the peridium surface were made radially and perpendicularly to the surface, halfway between the centre and the side of basidiomata. All microscopic features were drawn free hand using an Olympus Camera Lucida model U−DA fitted to the microscope cited above. For scanning electron microscopy (SEM), small fragments of dried hymenophore were mounted directly on to an SEM stub with double-sided tape. The samples were coated with gold for 60 seconds using SPI-Module Sputter Coater, examined and photographed at 15–20 kV with a FIB Quanta 200 3D scanning electron microscope (Thermo Fisher Scientific, United States).
DNA isolation, PCR amplification and DNA sequencing
Genomic DNA was extracted from fresh tissue preserved in CTAB or about 10–15 mg of dried specimens using a CTAB isolation procedure adapted from Doyle and Doyle (1990). The genes atp6, tef1 and rpb2 were amplified by polymerase chain reaction (PCR) technique. For the amplification of atp6, ATP6-1M40F and ATP6-2Mprimers were used (Raspé et al. 2016), with the following PCR programme: 2 min at 95 °C; 5 cycles of 45 s at 95 °C, 60 s at 42 °C, 30 s at 72 °C; 35 cycles of 20 s at 95 °C, 30 s at 55 °C, 30 s+1 s/cycle at 72 °C; 3 min at 72 °C. The primers EF1-983F and EF1-2218R (Rehner and Buckley 2005) were used to amplify tef1 and bRPB2-6F and bRPB2-7.1R primers (Matheny 2005) were used to amplify rpb2. PCR products were purified by adding 1 U of Exonuclease I and 0.5 U FastAP Alkaline Phosphatase (Thermo Scientific, St. Leon-Rot, Germany) and incubated at 37 °C for 1 h, followed by inactivation at 80 °C for 15 min. Sequencing was performed by Macrogen Inc. (Korea and The Netherlands) with PCR primers, except for atp6, for which universal primers M13F-pUC(-40) and M13F(-20) were used; for tef1, additional sequencing was performed with the two internal primers, EF1-1577F and EF1-1567R (Rehner and Buckley 2005).
Alignment and phylogeny inference
The sequences were assembled in GENEIOUS Pro v. 6.0.6 (Biomatters) and introns were removed prior to alignment based on the amino acid sequence of previously published sequences. All sequences, including sequences from GenBank, were aligned using MAFFT (Katoh and Standley 2013) on the server accessed at http://mafft.cbrc.jp/alignment/server/. Maximum Likelihood (ML) phylogenetic tree inference was performed using RAxML (Stamatakis 2006) on the CIPRES web server (RAxML-HPC2 on XSEDE; Miller et al. 2009). The phylogenetic tree was inferred by a single analysis with three partitions (one for each gene), using the GTRCAT model with 25 categories and three Chalciporus species were used as an outgroup. Statistical support of nodes was obtained with 1,000 bootstrap replicates.
Results
DNA analyses
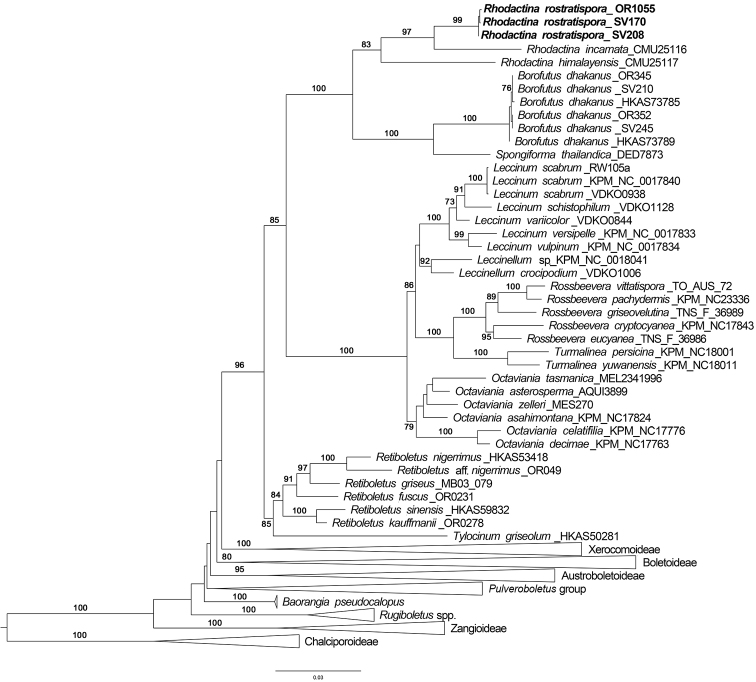
A total of 127 new sequences were generated and deposited in GenBank (Table 1). The alignment contained 157 taxa spread over the entire family Boletaceae and was 2429 characters long (TreeBase number 21933). The authors could not obtain tef1 and rpb2 sequences from R. incarnata (CMU25116) nor rpb2 sequence from R. himalayensis (CMU25117). The specimens were in relatively poor condition and genomic DNA was highly degraded. The 3-gene phylogram indicated that all selected collections of the new taxon R. rostratispora formed a monophyletic group with high bootstrap support sister to R. incarnata within the Rhodactina clade (Figure 1). The Rhodactina clade was sister to a clade composed of the genera Spongiforma Desjardin, Manfr. Binder, Roekring & Flegel and Borofutus Hosen & Zhu L. Yang, within the subfamily Leccinoideae G. Wu & Zhu L. Yang clade. Interestingly, the genera Rhodactina, Spongiforma and Borofutus formed a clade with 100% bootstrap support.
Table 1.
List of collections used for DNA analyses, with origin, GenBank accession numbers and reference(s).
| Species | Voucher | Origin | atp6 | tef1 | rpb2 | References |
|---|---|---|---|---|---|---|
| Afroboletus costatisporus | ADK4644 | Togo | KT823958 | KT824024 | KT823991 | Raspé et al. 2016 |
| Aureoboletus catenarius | HKAS54467 | China | – | KT990711 | KT990349 | Wu et al. 2016 |
| Aureoboletus duplicatoporus | HKAS50498 | China | – | KF112230 | KF112754 | Wu et al. 2014 |
| Aureoboletus gentilis | ADK4865 | Belgium | KT823961 | KT824027 | KT823994 | Raspé et al. 2016 |
| Aureoboletus moravicus | VDKO1120 | Belgium | MG212528 | MG212573 | MG212615 | This study |
| Aureoboletus nephrosporus | HKAS67931 | China | – | KT990720 | KT990357 | Wu et al. 2016 |
| Aureoboletus projectellus | AFTOL 713 | U.S.A. | DQ534604* | AY879116 | AY787218 | Binder and Hibbett 2006*; Binder et al. unpubl. |
| Aureoboletus thibetanus | HKAS76655 | China | – | KF112236 | KF112752 | Wu et al. 2014 |
| Aureoboletus tomentosus | HKAS80485 | China | – | KT990715 | KT990353 | Wu et al. 2016 |
| Aureoboletus viscosus | HKAS53398 | China | – | KF112238 | KF112755 | Wu et al. 2014 |
| Aureoboletus zangii | HKAS74766 | China | – | KT990726 | KT990363 | Wu et al. 2016 |
| Austroboletus cf. dictyotus | OR045 | Thailand | KT823966 | KT824032 | KT823999 | Raspé et al. 2016 |
| Austroboletus olivaceoglutinosus | HKAS57756 | China | – | KF112212 | KF112764 | Wu et al. 2014 |
| Austroboletus sp. | HKAS59624 | China | – | KF112217 | KF112765 | Wu et al. 2014 |
| Baorangia pseudocalopus | HKAS63607 | China | – | KF112167 | KF112677 | Wu et al. 2014 |
| Baorangia pseudocalopus | HKAS75739 | China | – | KJ184570 | KM605179 | Wu et al. 2015 |
| Boletellus aff. emodensis | OR061 | Thailand | KT823970 | KT824036 | KT824003 | Raspé et al. 2016 |
| Boletellus sp. | HKAS58713 | China | – | KF112307 | KF112759 | Wu et al. 2014 |
| Boletellus sp. | HKAS59536 | China | – | KF112306 | KF112758 | Wu et al. 2014 |
| Boletellus sp. | OR0621 | Thailand | MG212529 | MG212574 | MG212616 | This study |
| Boletus aereus | VDKO1055 | Belgium | MG212530 | MG212575 | MG212617 | This study |
| Boletus albobrunnescens | OR131 | Thailand | KT823973 | KT824039 | KT824006 | Raspé et al. 2016 |
| Boletus botryoides | HKAS53403 | China | – | KT990738 | KT990375 | Wu et al. 2016 |
| Boletus edulis | VDKO0869 | Belgium | MG212531 | MG212576 | MG212618 | This study |
| Boletus s.s. sp. | OR0446 | China | MG212532 | MG212577 | MG212619 | This study |
| Boletus erythropus | VDKO0690 | Belgium | KT823982 | KT824048 | KT824015 | Raspé et al. 2016 |
| Borofutus dhakanus | HKAS73789 | Bangladesh | – | JQ928576 | JQ928597 | Hosen et al. 2013 |
| Borofutus dhakanus | HKAS73785 | Bangladesh | – | JQ928577 | JQ928596 | Hosen et al. 2013 |
| Borofutus dhakanus | OR345 | Thailand | MG212533 | MG212578 | MG212620 | This study |
| Borofutus dhakanus | OR352 | Thailand | MG212534 | MG212579 | MG212621 | This study |
| Borofutus dhakanus | SV210 | Thailand | MG212535 | MG212580 | MG212622 | This study |
| Borofutus dhakanus | SV245 | Thailand | MG212536 | MG212581 | MG212623 | This study |
| Butyriboletus appendiculatus | VDKO0193b | Belgium | MG212537 | MG212582 | MG212624 | This study |
| Butyriboletus pseudoregius | VDKO0925 | Belgium | MG212538 | MG212583 | MG212625 | This study |
| Butyriboletus pseudospeciosus | HKAS63513 | China | – | KT990743 | KT990380 | Wu et al. 2016 |
| Butyriboletus roseoflavus | HKAS54099 | China | – | KF739779 | KF739703 | Wu et al. 2014 |
| Butyriboletus subsplendidus | HKAS50444 | China | – | KT990742 | KT990379 | Wu et al. 2016 |
| Butyroboletus cf. roseoflavus | OR230 | China | KT823974 | KT824040 | KT824007 | Raspé et al. 2016 |
| Caloboletus calopus | ADK4087 | Belgium | MG212539 | KJ184566 | KP055030 | This study; Zhao et al. 2014a; Zhao et al. 2014b |
| Caloboletus radicans | VDKO1187 | Belgium | MG212540 | MG212584 | MG212626 | This study |
| Caloboletus yunnanensis | HKAS69214 | China | – | KJ184568 | KT990396 | Zhao et al. 2014a; Wu et al. 2016 |
| Chalciporus aff. piperatus | OR586 | Thailand | KT823976 | KT824042 | KT824009 | Raspé et al. 2016 |
| Chalciporus africanus | JD517 | Cameroon | KT823963 | KT824029 | KT823996 | Raspé et al. 2016 |
| Chalciporus rubinus | AF2835 | Belgium | KT823962 | KT824028 | KT823995 | Raspé et al. 2016 |
| Chiua virens | OR0266 | China | MG212541 | MG212585 | MG212627 | This study |
| Chiua viridula | HKAS74928 | China | – | KF112273 | KF112794 | Wu et al. 2014 |
| Crocinoboletus cf. laetissimus | OR576 | Thailand | KT823975 | KT824041 | KT824008 | Raspé et al. 2016 |
| Cyanoboletus brunneoruber | OR0233 | China | MG212542 | MG212586 | MG212628 | This study |
| Cyanoboletus pulverulentus | RW109 | Belgium | KT823980 | KT824046 | KT824013 | Raspé et al. 2016 |
| Cyanoboletus sp. | OR0257 | China | MG212543 | MG212587 | MG212629 | This study |
| Fistulinella prunicolor | REH9502 | Australia | MG212544 | MG212588 | MG212630 | This study |
| Harrya chromapes | KPM NC17835 | Japan | KC552173 | JN378457 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Harrya moniliformis | HKAS49627 | China | – | KT990881 | KT990500 | Wu et al. 2016 |
| Heimioporus cf. mandarinus | OR0661 | Thailand | MG212545 | MG212589 | MG212631 | This study |
| Heimioporus japonicus | OR114 | Thailand | KT823971 | KT824037 | KT824004 | Raspé et al. 2016 |
| Heimioporus retisporus | HKAS52237 | China | – | KF112228 | KF112806 | This study |
| Heimioporus sp. | OR0218 | Thailand | MG212546 | MG212590 | MG212632 | This study |
| Hemileccinum depilatum | AF2845 | Belgium | MG212547 | MG212591 | MG212633 | This study |
| Hemileccinum impolitum | ADK4078 | Belgium | MG212548 | MG212592 | MG212634 | This study |
| Hemileccinum rugosum | HKAS84970 | China | – | KT990773 | KT990412 | Wu et al. 2016 |
| Hourangia cheoi | HKAS74744 | China | – | KF112285 | KF112772 | Wu et al. 2014 |
| Hourangia nigropunctata | HKAS 57427 | China | – | KP136927 | KP136978 | Zhu et al. 2015 |
| Hymenoboletus luteopurpureus | HKAS46334 | China | – | KF112271 | KF112795 | Wu et al. 2014 |
| Imleria badia | VDKO0709 | Belgium | KT823983 | KT824049 | KT824016 | Raspé et al. 2016 |
| Lanmaoa angustispora | HKAS74752 | China | – | KM605154 | KM605177 | Wu et al. 2015 |
| Lanmaoa asiatica | HKAS63603 | China | – | KM605153 | KM605176 | Wu et al. 2015 |
| Leccinellum crocipodium | VDKO1006 | Belgium | KT823988 | KT824054 | KT824021 | Raspé et al. 2016 |
| Leccinellum sp. | KPM-NC-0018041 | Japan | KC552165 | KC552094 | – | Orihara et al. 2016 |
| Leccinum scabrum | VDKO0938 | Belgium | MG212549 | MG212593 | MG212635 | This study |
| Leccinum scabrum | RW105a | Belgium | KT823979 | KT824045 | KT824012 | Raspé et al. 2016 |
| Leccinum scabrum | KPM-NC-0017840 | Scotland | KC552170 | JN378455 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Leccinum schistophilum | VDKO1128 | Belgium | KT823989 | KT824055 | KT824022 | Raspé et al. 2016 |
| Leccinum variicolor | VDKO0844 | Belgium | MG212550 | MG212594 | MG212636 | This study |
| Leccinum versipelle | KPM-NC-0017833 | Scotland | KC552172 | JN378454 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Leccinum vulpinum | KPM-NC-0017834 | Scotland | KC552171 | JN378456 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Mucilopilus castaneiceps | HKAS75045 | China | – | KF112211 | KF112735 | Wu et al. 2014 |
| Neoboletus brunneissimus | HKAS50538 | China | – | KM605150 | KM605173 | Wu et al. 2015 |
| Neoboletus brunneissimus | OR0249 | China | MG212551 | MG212595 | MG212637 | This study |
| Neoboletus junquilleus | AF2922 | France | MG212552 | MG212596 | MG212638 | This study |
| Neoboletus magnificus | HKAS54096 | China | – | KF112149 | KF112654 | Wu et al. 2014 |
| Neoboletus venenatus | HKAS63535 | China | – | KT990807 | KT990448 | Wu et al. 2016 |
| Octaviania asahimontana | KPM-NC17824 | Japan | KC552154 | JN378430 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Octaviania asterosperma | AQUI3899 | Italy | KC552159 | KC552093 | – | Orihara et al. 2016 |
| Octaviania celatifilia | KPM-NC17776 | Japan | KC552147 | JN378416 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Octaviania decimae | KPM-NC17763 | Japan | KC552145 | JN378409 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Octaviania tasmanica | MEL2341996 | Australia | KC552156 | JN378436 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Octaviania zelleri | MES270 | U.S.A. | KC552161 | JN378440 | – | Orihara et al. 2016; Orihara et al. 2012 |
| Phylloporus brunneiceps | OR050 | Thailand | KT823968 | KT824034 | KT824001 | Raspé et al. 2016 |
| Phylloporus castanopsidis | OR052 | Thailand | KT823969 | KT824035 | KT824002 | Raspé et al. 2016 |
| Phylloporus imbricatus | HKAS68642 | China | – | KF112299 | KF112786 | Wu et al. 2014 |
| Phylloporus luxiensis | HKAS75077 | China | – | KF112298 | KF112785 | Wu et al. 2014 |
| Phylloporus yunnanensis | OR0448 | China | MG212554 | MG212598 | MG212640 | This study |
| Porphyrellus castaneus | OR0241 | China | MG212555 | MG212599 | MG212641 | This study |
| Porphyrellus porphyrosporus | MB97-023 | Germany | DQ534609 | GU187734 | GU187800 | Binder and Hibbett 2006; Binder et al. 2010 |
| Pulveroboletus aff. ravenelii | ADK4360 | Togo | KT823957 | KT824023 | KT823990 | Raspé et al. 2016 |
| Pulveroboletus aff. ravenelii | ADK4650 | Togo | KT823959 | KT824025 | KT823992 | Raspé et al. 2016 |
| Pulveroboletus aff. ravenelii | HKAS53351 | China | – | KF112261 | KF112712 | Wu et al. 2014 |
| Pulveroboletus fragrans | OR673 | Thailand | KT823977 | KT824043 | KT824010 | Raspé et al. 2016 |
| Pulveroboletus ravenelii | REH2565 | U.S.A. | KU665635 | KU665636 | KU665637 | Raspé et al. 2016 |
| Pulveroboletus sp. | HKAS74933 | China | – | KF112262 | KF112713 | Wu et al. 2014 |
| Retiboletus aff. nigerrimus | OR049 | Thailand | KT823967 | KT824033 | KT824000 | Raspé et al. 2016 |
| Retiboletus fuscus | OR0231 | China | MG212556 | MG212600 | MG212642 | This study |
| Retiboletus griseus | MB03-079 | U.S.A. | KT823964 | KT824030 | KT823997 | Raspé et al. 2016 |
| Retiboletus kauffmanii | OR0278 | China | MG212557 | MG212601 | MG212643 | This study |
| Retiboletus nigerrimus | HKAS53418 | China | – | KT990824 | KT990462 | Wu et al. 2016 |
| Retiboletus sinensis | HKAS59832 | China | – | KT990827 | KT990464 | Wu et al. 2016 |
| Rhodactina himalayensis | CMU25117 | Thailand | MG212558 | MG212602, MG212603 | – | This study |
| Rhodactina incarnata | CMU25116 | Thailand | DQ328982 | – | – | Yang et al. 2006 |
| Rhodactina rostratispora | OR1055 | Thailand | MG212559 | MG212604 | MG212644 | This study |
| Rhodactina rostratispora | SV170 | Thailand | MG212560 | MG212605 | MG212645 | This study |
| Rhodactina rostratispora | SV208 | Thailand | MG212561 | MG212606 | MG212646 | This study |
| Rossbeevera cryptocyanea | KPM-NC17843 | Japan | KT581441 | KC552072 | – | Orihara et al. 2016 |
| Rossbeevera eucyanea | TNS-F-36986 | Japan | KC552115 | KC552068 | – | Orihara et al. 2016 |
| Rossbeevera griseovelutina | TNS-F-36989 | Japan | KC552124 | KC552076 | – | Orihara et al. 2016 |
| Rossbeevera pachydermis | KPM-NC23336 | New Zealand | KJ001064 | KP222912 | – | Orihara et al. 2016 |
| Rossbeevera vittatispora | TO-AUS-72 | Australia | KC552108 | KC552065 | – | Orihara et al. 2016 |
| Royoungia reticulata | HKAS52253 | China | – | KT990786 | KT990427 | Wu et al. 2016 |
| Royoungia rubina | HKAS53379 | China | – | KF112274 | KF112796 | Wu et al. 2014 |
| Rubroboletus legaliae | VDKO0936 | Belgium | KT823985 | KT824051 | KT824018 | Raspé et al. 2016 |
| Rubroboletus satanas | VDKO0968 | Belgium | KT823986 | KT824052 | KT824019 | Raspé et al. 2016 |
| Rubroboletus sinicus | HKAS56304 | China | – | KJ619483 | KP055031 | Zhao et al. 2014a; Zhao et al. 2014b |
| Rugiboletus brunneiporus | HKAS83209 | China | – | KM605144 | KM605168 | Wu et al. 2015 |
| Rugiboletus extremiorientalis | HKAS76663 | China | – | KM605147 | KM605170 | Wu et al. 2015 |
| Rugiboletus extremiorientalis | OR0406 | Thailand | MG212562 | MG212607 | MG212647 | This study |
| Spongiforma thailandica | DED7873 | Thailand | MG212563 | KF030436* | MG212648 | Nuhn et al. 2013*; This study |
| Strobilomyces atrosquamosus | HKAS55368 | China | – | KT990839 | KT990476 | Wu et al. 2016 |
| Strobilomyces echinocephalus | OR0243 | China | MG212564 | MG212608 | MG212649 | This study |
| Strobilomyces floccopus | RW103 | Belgium | KT823978 | KT824044 | KT824011 | Raspé et al. 2016 |
| Strobilomyces mirandus | OR115 | Thailand | KT823972 | KT824038 | KT824005 | Raspé et al. 2016 |
| Strobilomyces sp. | OR0259 | China | MG212565 | MG212609 | MG212650 | This study |
| Strobilomyces sp. | OR0778 | Thailand | MG212566 | MG212610 | MG212651 | This study |
| Strobilomyces verruculosus | HKAS55389 | China | – | KF112259 | KF112813 | Wu et al. 2014 |
| Suillellus luridus | VDKO0241b | Belgium | KT823981 | KT824047 | KT824014 | Raspé et al. 2016 |
| Suillellus subamygdalinus | HKAS53641 | China | – | KT990841 | KT990478 | Wu et al. 2016 |
| Sutorius australiensis | REH9441 | Australia | MG212567 | JQ327032* | MG212652 | Halling et al. 2012*; This study |
| Sutorius eximius | REH9400 | U.S.A. | MG212568 | JQ327029* | MG212653 | Halling et al. 2012*; This study |
| Turmalinea persicina | KPM-NC18001 | Japan | KC552130 | KC552082 | – | Orihara et al. 2016 |
| Turmalinea yuwanensis | KPM-NC18011 | Japan | KC552138 | KC552089 | – | Orihara et al. 2016 |
| Tylocinum griseolum | HKAS50281 | China | – | KF112284 | KF112730 | Wu et al. 2014 |
| Tylopilus atripurpureus | HKAS50208 | China | – | KF112283 | KF112799 | Wu et al. 2014 |
| Tylopilus balloui s.l. | OR039 | Thailand | KT823965 | KT824031 | KT823998 | Raspé et al. 2016 |
| Tylopilus felleus | VDKO0992 | Belgium | KT823987 | KT824053 | KT824020 | Raspé et al. 2016 |
| Tylopilus sp. | OR0252 | China | MG212569 | MG212611 | MG212654 | This study |
| Tylopilus sp. | OR0542 | Thailand | MG212570 | MG212612 | MG212655 | This study |
| Tylopilus vinaceipallidus | OR0137 | China | MG212571 | MG212613 | MG212656 | This study |
| Veloporphyrellus alpinus | HKAS57490 | China | JX984514 | JX984549 | – | Li et al. 2014 |
| Veloporphyrellus conicus | CFMR BZ1670 | Belize | JX984520 | JX984555 | – | Li et al. 2014 |
| Veloporphyrellus pseudovelatus | HKAS52258 | China | JX984517 | JX984551 | – | Li et al. 2014 |
| Veloporphyrellus velatus | HKAS63668 | China | JX984523 | JX984554 | – | Li et al. 2014 |
| Xerocomellus chrysenteron | VDKO0821 | Belgium | KT823984 | KT824050 | KT824017 | Raspé et al. 2016 |
| Xerocomellus cisalpinus | ADK4864 | Belgium | KT823960 | KT824026 | KT823993 | Raspé et al. 2016 |
| Xerocomus fulvipes | HKAS76666 | China | – | KF112292 | KF112789 | Wu et al. 2014 |
| Xerocomus subtomentosus | VDKO0987 | Belgium | MG212572 | MG212614 | MG212657 | This study |
| Zangia citrina | HKAS52684 | China | HQ326850 | HQ326872 | – | Li et al. 2011 |
| Zangia olivacea | HKAS55830 | China | HQ326855 | HQ326874 | – | Li et al. 2011 |
| Zangia olivaceobrunnea | HKAS52275 | China | HQ326856 | HQ326875 | – | Li et al. 2011 |
| Zangia roseola | HKAS51137 | China | HQ326858 | HQ326877 | – | Li et al. 2011 |
Figure 1.
Maximum likelihood phylogenetic tree inferred from the three-gene dataset (atp6, rpb2, tef1), including Rhodactina rostratispora and selected Boletaceae. The three Chalciporus species were used as outgroup taxa. Most of the taxa not belonging to the subfamily Leccinoideae were collapsed into subfamilies or similar level clade (i.e. Pulveroboletus group). Bootstrap support values > 70% are shown above branches.
Taxonomy
Key to the species of Rhodactina
| 1 | Basidiospores with a markedly prominent hilar appendage 2.5–5 µm long and 3.5–5 µm wide with a terminal hilum, spore size 12–16 × 10–14 µm | R. rostratispora sp. nov. |
| – | Basidiospores without markedly prominent hilar appendage or with short to nearly truncate hilar appendage up to 1.5 µm long and 1.5 µm wide | 2 |
| 2 | Basidiospores bearing large (5)6–7(8) longitudinal ridges, 3–4 µm wide, up to 5 µm tall, dark violet in 5 % KOH, spore size 15–20 × 12.5–18 µm | R. himalayensis |
| – | Basidiospores bearing (7)8–9(10) longitudinal ridges, 2–3 µm wide, up to 3 µm tall, slightly reddish to purplish yellow in 5 % KOH, spore size 10–13 × 10–12 µm | R. incarnata |
Rhodactina rostratispora
Vadthanarat, Raspé & Lumyong sp. nov.
822126
Figure 2.
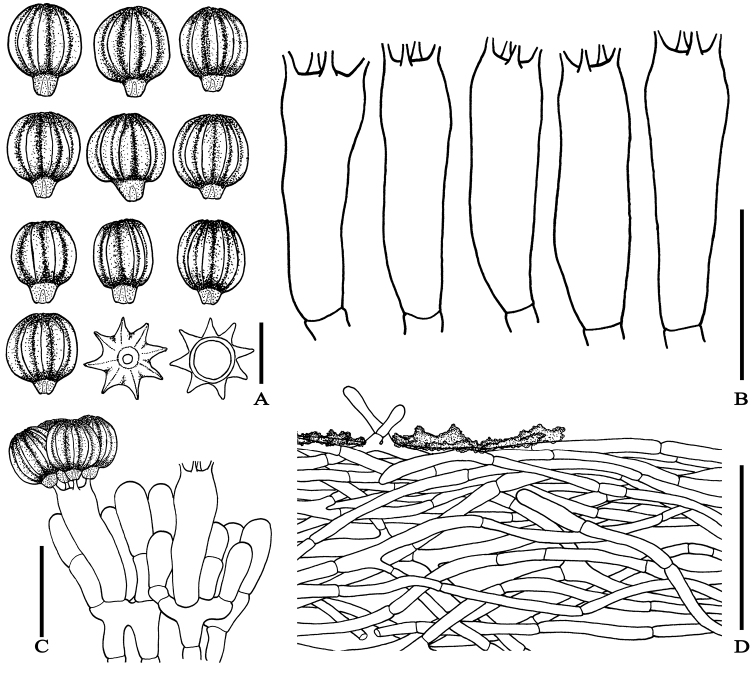
Basidiomata of Rhodactina rostratispora A S. Vadthanarat 170 (holotype) B S. Vadthanarat 206 C S. Vadthanarat 208 D O. Raspé 1055 E S. Vadthanarat 406, showing one basidioma (white arrow) that had a strong fruity alcoholic smell F Hymenophore turned dark purple to greyish violet with 5% KOH (white arrow). Scale bars: A–E = 1 cm; F =0.5 cm.
Figure 3.
Microscopic features of Rhodactina rostratispora A Basidiospores in side view, polar view and optical section B Basidia C Hymenium showing basidia and basidioles D Peridiopellis covered with some encrustations. All drawings were made from the type. Scale bars: A = 10 µm; B–C = 20 µm; D = 50 µm.
Figure 4.
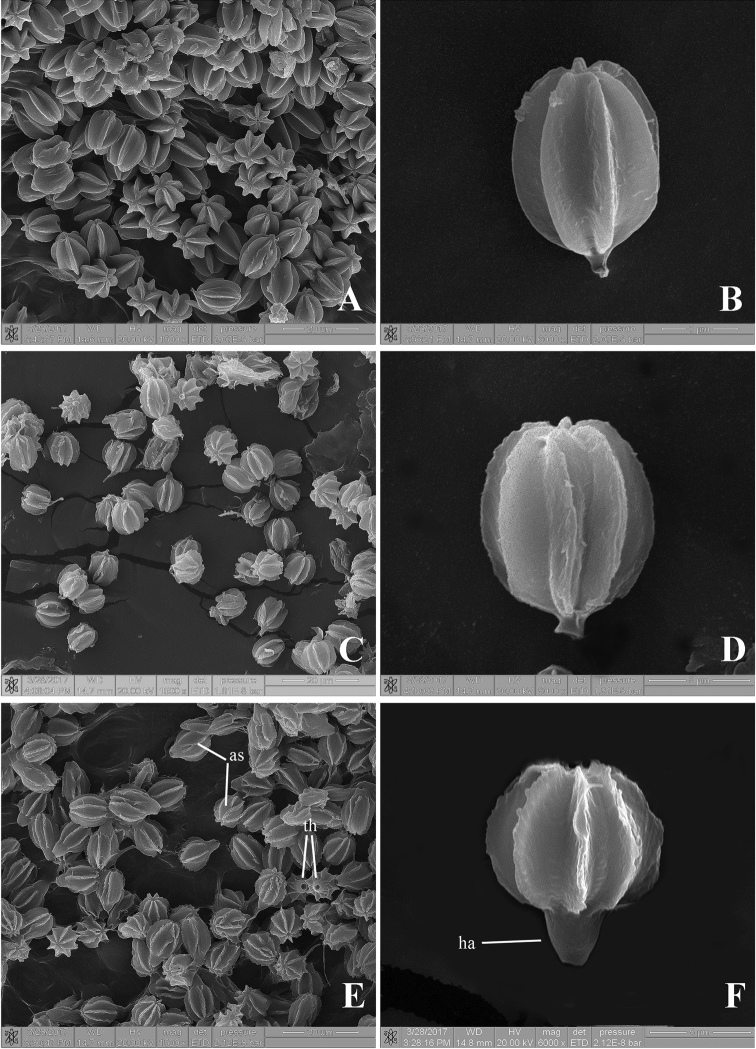
Scanning electron micrographs of basidiospores A–B Rhodactina himalayensis (CMU25117) showing the basidiospores with 6–7 longitudinal ridges C–D Rhodactina incarnata (CMU25116, holotype) showing the basidiospores with 8–9 longitudinal ridges E–F Rhodactina rostratispora (O. Raspé 1055) showing the basidiospores with 8–9 longitudinal ridges, the wide and prominent hilar appendage (ha), a terminal hilum (th) and anastomosing ridges in some spores (as).
Type.
THAILAND, Ubon Ratchathani Province, Trakan Phuet Phon District, Don Khok Tam Lae community forest, 15°35'46"N, 105°06'38"E, elev. 150 m., 28 July 2015, S. Vadthanarat 170, (holotype: CMUB!; isotype: BR!).
Etymology.
From Latin “rostrati–” meaning having beaked prow or a solid projection and “spora” meaning spores, referring to the basidiospores having a markedly prominent and large hilar appendage.
Description.
Basidiomata small to medium-sized 0.8–2.5(4.5) cm diam., subglobose to ovoid with a rudimentary elongated basal attachment, with greyish white to pale brown rhizoids at the base and going up along the surface of basidiomata to about half of the height. Peridium surface (outer peridium) fibrillose to arachnoid, off-white to pinkish white (7A2–3 to 9A2), dull, moist, cracked in places. Peridium very thin, 0.1–0.2(0.4) mm thick. Hymenophore cartilaginous, completely enclosed, whitish orange to reddish orange (7A3–4 to 8A5–6) at first becoming orangey red to red (9D–E8 to 10D–E8) with age, then dark red when very old, irregular; Stipe-columella absent. Taste fungoid. Odour absent when young, very strongly fruity alcoholic when old.
Macrochemical reactions: hymenophore turned dark purplish (15F8) to greyish violet (19D3) with 5% KOH, slightly greyish violet (19D3) with NH4OH.
Basidiospores [404/8/8] (11.5–)12–13.6–15(–16) × (10–)10.5–11.7–13(–14), Q = (1–)1.04–1.16–1.3(–1.4), from the holotype, (12–)12–13.5–15.2(–16) × (10–)10–11.6–13.2(–14) µm, Q = (1–)1–1.02–1.33(–1.4), N = 50, ellipsoid to broadly ellipsoid with longitudinal ridges, stellate in polar-view, thick-walled (1–1.5 µm thick), yellowish to orangey hyaline to reddish yellow at first, reddish to brownish yellow with age in water, slightly purplish and slightly more reddish to brownish in 5% KOH, slightly purplish hyaline in NH4OH, slightly dextrinoid to dextrinoid in Melzer’s reagent; ornamentation (7)8–9 solid ridges regularly and longitudinally arranged under light microscope, sometimes anastomosing under SEM, 2–3 µm tall and 2–2.5 µm wide at the base; hilar appendage prominent, 2.5–5 µm long with a terminal hilum. Basidia 4–spored, (26–)26.1–32.3–36(–36) × (8–)8–9.5–11(–11) µm (n = 20; from holotype only), clavate to cylindrical, hyaline in water, 5% KOH and NH4OH, yellowish hyaline in Melzer’s reagent; sterigmata broken by spore release, stout, 3–4 µm long. Cystidia none observed. Hymenophoral trama 60–130 µm thick, irregular, with a narrow, central layer of subparallel to loosely interwoven, 3–7(8) µm wide, thin-walled hyphae, slightly gelatinised, hyaline in water, 5% KOH and NH4OH. Peridiopellis a tomentum 45–120 µm thick, poorly differentiated, composed of thin-walled, 3–10 µm wide hyphae, anastomosing at places and covered with yellowish brown incrustations on the surface at places, otherwise hyaline in water, 5% KOH and NH4OH, inamyloid. Clamp connections not seen in any of the tissues.
Habit and habitat.
Subepigeal, solitary to gregarious (4–7 basidiomata), or fasciculate by 2–5 basidiomata, on sandy soil in dipterocarp forest dominated by Dipterocarpus tuberculatus, D. intricatus, D. obtusifolius, Shorea obtusa, S. siamensis and Eucalyptus sp.
Known distribution.
Currently found only from Ubon Ratchathani province, northeastern Thailand.
Additional specimens examined.
Rhodactina rostratispora.—THAILAND, Ubon Ratchathani Province, Trakan Phuet Phon District, Don Khok Tam Lae community forest, 15°35'40.2"N–105°06'37.8"E, elev. 150 m., 28 July 2015, S. Vadthanarat 169, (CMUB, BR); ibid. 15°35'41.5"N–105°06'35.4"E, elev. 150 m., 28 July 2015, O. Raspé 1055, (CMUB, BR); ibid. 15°35'48.3"N –105°06'35.9"E, elev. 150 m., 6 August 2015, S. Vadthanarat 206, (CMUB, BR); ibid. 15°35'52.4"N–105°06'41.2"E, elev. 150 m., 6 August 2015, S. Vadthanarat 208, (CMUB, BR); ibid. 15°35'56.1"N–105°06'38.9"E, elev. 150 m., 6 August 2015, S. Vadthanarat 212, (CMUB, BR); ibid. 15°36'2.6"N–105°06'36.7"E, elev. 150 m., 14 May 2017, S. Vadthanarat 376, (CMUB, BR); Ban Huay Fai community forest, 15°32'42.7"N–105°10'16.3"E, elev. 160 m., 15 July 2017, S. Vadthanarat 406, (CMUB, BR).
R. himalayensis. – THAILAND, Chiang Mai Province, Doi Suthep-Pui National Park, forest behind Channel 9 TV station, 4 August 2000, Saisamorn Lumyong, Pipob Lumyong, Rarunee Sanmee and B. Dell 2254 (CMU25117).
R. incarnata. – THAILAND, Chiang Mai Province, Sanpatong District, Mae Wang, Conservation forest, Sanpatong-Ban Guard Rd., 24 July 2002, Saisamorn Lumyong, Pipob Lumyong, Rarunee Sanmee and Zhu L. Yang 45209 (CMU25116; holotype).
Remarks.
Rhodactina rostratispora is characterised by its basidiospores having a markedly prominent hilar appendage (2.5–5 µm long, 3.5–5 µm wide), with a terminal hilum; ornamentation consisting of (7)8–9 longitudinal ridges, and (11.5–)12–13.6–15(–16) × (10–)10.5–11.7–13(–14) µm. R. himalayensis has larger basidiospores (15–20 × 12.5–18 µm) without prominent hilar appendage, with fewer [(5)6–7(8)], broader ridges, while R. incarnata has a similar spore size (10–13 × 10–12 µm) and the same number of spore ridges [(7)8–9(10)] as the new species, but it does not have the prominent hilar appendage.
In one R. rostratispora specimen (S. Vadthanarat 208), abnormal spores were observed. Those spores were elongated, 21–24 × 4–8 µm, thick-walled, narrowly fusiform to bacilliform, with or without longitudinal ridges, more or less constricted in the middle. They were usually found attached to apparently normal basidia with four sterigmata.
Discussion
Morphologically, the new species R. rostratispora is characterised by its ridged basidiospores having a markedly prominent hilar appendage with a terminal hilum, which is not found in other Rhodactina species (Pegler and Young 1989, Yang et al. 2006). However, ridged basidiospores having a prominent hilar appendage are found in some other sequestrate Boletaceae in the genus Turmalinea Orihara & N. Maek and Rossbeevera, including T. persicina Orihara, T. chrysocarpa Orihara & Z.W. Ge, T. mesomorpha Orihara, Ro. paracyanea Orihara and Ro. cryptocyanea Orihara. The basidiospores of those species have a long pointed hilar appendage 4.5–6 µm (Orihara et al. 2016) but are not as wide as in R. rostratispora (2.5–5 µm long, 3.5–5 µm wide) and also their hilar appendage lacks a terminal hilum. Macroscopically, those species differ from R. rostratispora in that both Rossbeevera and Turmalinea have basidiomata often turning blue to greenish blue when bruised, which has never been reported in any Rhodactina species (Pegler and Young 1989, Yang et al. 2006). Moreover, the colour of mature hymenophore of Turmalinea and Rossbeevera species are dark brown or blackish brown (Lebel et al. 2012, Orihara et al. 2016) not red or dark red like in Rhodactina.
The phylogenetic analyses also support the placement of the new taxon in the genus Rhodactina, with R. incarnata being the closest species. The phylogenetic tree also showed that Rhodactina is sister to a clade composed of Spongiforma and Borofutus within the subfamily Leccinoideae, with 100% bootstrap support. According to Wu et al. (2016), there are 10 genera in the sub-family Leccinoideae including Borofutus, Chamonixia Rolland, Leccinum Gray, Leccinellum Bresinsky & Manfr. Binder, Octaviania Vittad, Pseudoaustroboletus Y.C. Li & Zhu L. Yang, Retiboletus Manfr. Binder & Bresinsky, Rossbeevera T. Lebel & Orihara & N. Maek, Spongiforma and Tylocinum Yan C. Li & Zhu L. Yang. The phylogenetic analyses infer that Rhodactina is the eleventh genus in the subfamily.
In the examination of R. rostratispora, it was found that the hymenophore turned dark purplish to greyish violet with 5% KOH. Interestingly, all of the genera in subfamily Leccinoideae that turn purple to violet with aqueous KOH solution, namely Rhodactina, Borofutus and Spongiforma, are grouped in one clade with 100% bootstrap support. All of the species in the clade share the characteristic of the basidiospores turning more or less purplish, purplish red to violet grey in aqueous KOH solution (Desjardin et al. 2009, Hosen et al. 2013). Spongiforma squarepantsii Desjardin, Peay & T.D. Bruns, which was described from Malaysia, was not included in these analyses, but the original description of this species also mentioned that its basidiospores turn pale lilac grey in 3% KOH (Desjardin et al. 2011). A chemical reaction with KOH was observed not only with basidiospores, but also on the hymenophore (Desjardin et al. 2009). The reaction to 5% KOH has been observed on fresh basidiomata of Borofutus dhakanus Hosen & Zhu L. Yang which is an epigeous species and the only currently known species of this genus. The colour reaction of pileus and pileus context, which turned pinkish blue to purplish blue, was different from that of the stipe and stipe context, which turned yellowish green to olive green. This variation in colour of the reaction to 5% KOH was not mentioned in the original description of the species (Hosen et al. 2013). Therefore, this chemical character is very useful for the identification of boletes belonging to this group. Other taxa that have been reported to show similar colour reactions to KOH and would, therefore, belong to this group, include Austroboletus longipes (Massee) Wolfe, Austroboletus malaccensis (Pat. & C.F. Baker) Wolfe and Austroboletus tristis (Pat. & C.F. Baker) Wolfe (Corner 1972, Horak 2011).
Some basidiomata of R. rostratispora were old when collected, with dark red hymenophore and had a very strong fruity, alcoholic odour. The odour seems to be present in old basidiomata only (S. Vadthanarat 212 and one basidiomata of S. Vadthanarat 406). One possible explanation to the alcoholic smell is that sterigmata are broken from spore release and any remaining cytoplasm in the basidia could leak into the cavities of the hymenophore and be fermented. Fermentation by yeasts might be possible due to the cracking of the peridium, allowing contact of the hymenophore cavities with ambient air. As mammals and marsupials are known to be the main spore dispersal vectors of truffle-like fungi (e.g. Lamont et al. 1985, Cázares and Trappe 1994, Vernes and Dunn 2009), the strong alcoholic smell could facilitate detection and entice consumption of the basidiomata by mammals and thus help spore dispersal.
The three Rhodactina species were found only in dipterocarp forest between 100 to 600 m above sea level in India, northern and northeastern Thailand (Pegler and Young 1989, Yang et al. 2006). They presumably form ectomycorrhizal associations with trees of the genera Dipterocarpus and Shorea (Dipterocarpaceae). However, in the forest where the new species was found, some scattered Eucalyptus trees were also observed. As Eucalyptus species have been reported to be ectomycorrhizal trees (e.g. Giachini et al. 2000, Ducousso et al. 2012, Garrett Kluthe et al. 2016), the Eucalyptus trees found in the forest could also possibly be host of R. rostratispora. However, Eucalyptus is not indigenous to Thailand; several species have been planted since the early 1900s (Luangviriyasaeng 2003). As Rhodactina species seem to be indigenous to Thailand and Eucalyptus not, they are less likely to be ectomycorrhizal partners. Further study is needed, however, to confirm the range of ectomycorrhizal host tree species of R. rostratispora. Borofutus and Spongiforma, the most closely related genera of Rhodactina, are also ectomycorrhizal associates with trees in Dipterocarpaceae. The only known Borofutus species, B. dhakanus is ectomycorrhizal with Shorea robusta (Hosen et al. 2013). As for Spongiforma species, S. thailandica was reported as associated with Dipterocarpus sp. and Shorea sp. in primary forest while S. squarepantsii was reported as associated with unidentified dipterocarp trees (Desjardin et al. 2009, Desjardin et al. 2011).
Supplementary Material
Acknowledgments
Financial support from the Graduate School, Chiang Mai University, is appreciated. The work was partly supported by a TRF Research Team Association Grant (RTA 5880006) to SL and OR and by the Higher Education Research Promotion and the Thai Centre of Excellence on Biodiversity (BDC-PG2-159013) and Center of Excellence in Bioresources for Agriculture, Industry and Medicine, Faculty of Science, Chiang Mai University. OR is grateful to the Fonds National de la Recherche Scientifique (Belgium) for travel grants. The authors are grateful to Dennis Desjardin and Roy Halling for the loan of specimens. The comments of Roy Halling and Roy Watling helped improving the article and are gratefully acknowledged.
Citation
Vadthanarat S, Raspé O, Lumyong S (2018) Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys 29: 63–80. https://doi.org/10.3897/mycokeys.29.22572
References
- Binder M, Hibbett DS. (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98: 971–981. https://doi.org/10.1080/15572536.2006.11832626 [DOI] [PubMed] [Google Scholar]
- Binder M, Larsson KH, Matheny Hibbett DS. (2010) Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 102: 865–880. https://doi.org/10.3852/09-288 [DOI] [PubMed] [Google Scholar]
- Cázares E, Trappe JM. (1994) Spore dispersal of ectomycorrhizal fungi on a glacier forefront by mammal mycophagy. Mycologia 86: 507–510. https://doi.org/10.2307/3760743 [Google Scholar]
- Chandrasrikul A, Suwanarit P, Sangwanit U, Lumyong S, Payapanon A, Sanoamuang N, Pukahuta C, Petcharat V, Sardsud U, Duengkae K, Klinhom U, Thongkantha S, Thongklam S. (2011) Checklist of Mushrooms (Basidiomycetes) in Thailand. Office of Natural Resources and Environmental Policy and Planning, Bangkok, Thailand, 1–448.
- Choeyklin R, Boonpratuang T, Sommai S, Somrithipol S. (2012) https://doi.org/10.5248/120.149
- Corner EJH. (1972) Boletus in Malaysia. Gov. Printer, Singapore, 1–263.
- Desjardin DE, Binder M, Roekring S, Flegel T. (2009) Spongiforma, a new genus of gasteroid boletes from Thailand. Fungal Diversity 37: 1–8. [Google Scholar]
- Desjardin DE, Peay KG, Bruns TD. (2011) Spongiforma squarepantsii, a new species of gasteroid bolete from Borneo. Mycologia 103(5): 1119–1123. https://doi.org/10.3852/10-433 [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13–15. [Google Scholar]
- Ducousso M, Duponnois R, Thoen D, Prin Y. (2012) Diversity of ectomycorrhizal fungi associated with Eucalyptus in Africa and Madagascar. International Journal of Forestry Research 2012: 1–10. https://doi.org/10.1155/2012/450715 [Google Scholar]
- Garrett Kluthe B, Ben Hassine Ben Ali M, Nelsen DJ, Stephenson SL. (2016) A preliminary study of the ectomycorrhizal fungi associated with introduced Eucalyptus in Kenya. Mycosphere 7(1): 81–86. [Google Scholar]
- Giachini AJ, Oliveira VL, Castellano MA, Trappe JM. (2000) Ectomycorrhizal fungi in Eucalyptus and Pinus plantations in Southern Brazil. Mycologia 92(6): 1166–1177. https://doi.org/10.2307/3761484 [Google Scholar]
- Halling RE, Desjardin DE, Fechner N, Arora D, Soytong K, Dentinger BTM. (2014) New porcini (Boletus sect. Boletus) from Australia and Thailand. Mycologia 106: 830–834. https://doi.org/10.3852/13-340 [DOI] [PubMed] [Google Scholar]
- Halling RE, Nuhn M, Fechner NA, Osmundson TW, Soytong K, Arora D, Hibbett DS, Binder M. (2012) Sutorius: a new genus for Boletus eximius. Mycologia 104(4): 951–961. https://doi.org/10.3852/11-376 [DOI] [PubMed] [Google Scholar]
- Horak E. (2011) Revision of Malaysian Species of Boletales s.l. (Basidiomycota) Described by Corner EJH (1972, 1974). Forest Research Institute and Ministry of Natural Resources and Environment, Malaysia, 1–283.
- Hosen MI, Feng B, Wu G, Zhu XT, Li YC, Yang ZL. (2013) Borofutus, a new genus of Boletaceae from tropical Asia: phylogeny, morphology and taxonomy. Fungal Diversity 58: 215–226. https://doi.org/10.1007/s13225-012-0211-8 [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. https://doi.org/10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. (1978) Methuen Handbook of Colour. 3rd ed. Eyre Methuen Ltd, London, 1–252.
- Kretzer AM, Bruns TD. (1999) Use of atp6 in fungal phylogenetics: an example from the Boletales. Molecular Phylogenetics and Evolution 13: 483–492. https://doi.org/10.1006/mpev.1999.0680 [DOI] [PubMed] [Google Scholar]
- Lamont BB, Ralph CS, Christensen PES. (1985) Mycophagous marsupials as dispersal agents for ectomycorrhizal fungi on Eucalyptus calophylla and Gastrolobium bilobum. New Phytologist 101: 651–656. https://doi.org/10.1111/j.1469-8137.1985.tb02870.x [Google Scholar]
- Lebel T, Orihara T, Maekawa N. (2012) The sequestrate genus Rosbeeva T. Lebel & Orihara gen. nov. (Boletaceae) from Australasia and Japan: new species and new combinations. Fungal Diversity 52: 49–71. https://doi.org/10.1007/s13225-011-0109-x [Google Scholar]
- Li YC, Feng B, Yang ZL. (2011) Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Diversity 49: 125–143. https://doi.org/10.1007/s13225-011-0096-y [Google Scholar]
- Li YC, Ortiz-Santana B, Zeng NK, Feng B. (2014) Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycologia 106(2): 291–306. https://doi.org/10.3852/106.2.291 [DOI] [PubMed] [Google Scholar]
- Luangviriyasaeng V. (2003) Eucalypt planting in Thailand. In: Turnbull JW. (Ed.) Eucalypts in Asia. Proceedings of an international conference held in Zhanjiang, Guangdong, People’s Republic of China, 7–11 April 2003. ACIAR Proceedings no. 111. Australian Centre for International Agricultural Research, Canberra, 28–31.
- Matheny PB. (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Molecular Phylogenetics and Evolution 35: 1–20. https://doi.org/10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. (2009) The CIPRES portals. CIPRES. Available at: http://www.phylo.org/portal2/home
- Neves MA, Binder M, Halling R, Hibbett D, Soytong K. (2012) The phylogeny of selected Phylloporus species inferred from NUC-LSU and ITS sequences, and descriptions of new species from the Old World. Fungal Diversity 55(1): 109–123. https://doi.org/10.1007/s13225-012-0154-0 [Google Scholar]
- Nuhn ME, Binder M, Taylor AFS, Halling RE, Hibbett DS. (2013) Phylogenetic overview of the Boletineae. Fungal Biology 117: 479–511. https://doi.org/10.1016/j.funbio.2013.04.008 [DOI] [PubMed] [Google Scholar]
- Orihara T, Lebel T, Ge Z-W, Smith ME, Maekawa N. (2016) Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of ITS minisatellite-like insertions for molecular identification. Persoonia 37: 173–198. https://doi.org/10.3767/003158516X691212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihara T, Smith ME, Shimomura N, Iwase K, Maekawa N. (2012) Diversity and systematics of the sequestrate genus Octaviania in Japan: two new subgenera and eleven new species. Persoonia 28: 85–112. https://doi.org/10.3767/003158512X650121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegler DN, Young TWK. (1989) Rhodactina himalayensis gen. et sp. nov. (Gautieriaceae) from India. Opera Botanica 100: 201–206. [Google Scholar]
- Raspé O, Vadthanarat S, De Kesel A, Degreef J, Hyde KD, Lumyong S. (2016) Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycological Progress 15: 38. https://doi.org/10.1007/s11557-016-1179-7
- Rehner SA, Buckley E. (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Smith ME, Amses KR, Elliott TF, Obase K, Aime MC, Henkel TW. (2015) New sequestrate fungi from Guyana: Jimtrappea guyanensis gen. sp. nov., Castellanea pakaraimophila gen. sp. nov., and Costatisporus cyanescens gen. sp. nov. (Boletaceae, Boletales). IMA Fungus 6(2): 297–317. https://doi.org/10.5598/imafungus.2015.06.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2006) RAxML-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. https://doi.org/10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Thiers B (continuously updated) Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/
- Vernes K, Dunn L. (2009) Mammal mycophagy and fungal spore dispersal across a steep environmental gradient in eastern Australia. Austral Ecology 34: 69–76. https://doi.org/10.1111/j.1442-9993.2008.01883.x [Google Scholar]
- Wu G, Feng B, Xu J, Zhu XT, Li YC, Zeng NK, Hosen MI, Yang ZL. (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Diversity 69: 93–115. https://doi.org/10.1007/s13225-014-0283-8 [Google Scholar]
- Wu G, Li YC, Zhu XT, Zhao K, Han LH, Cui YY, Li F, Xu JP, Yang ZL. (2016) One hundred noteworthy boletes from China. Fungal Diversity 81: 25–188. https://doi.org/10.1007/s13225-016-0375-8 [Google Scholar]
- Wu G, Zhao K, Li YC, Zeng NK, Feng B, Halling RE, Yang ZL. (2015) Four new genera of the fungal family Boletaceae. Fungal Diversity 81: 1–24. https://doi.org/10.1007/s13225-015-0322-0 [Google Scholar]
- Yang ZL, Trappe JM, Binder M, Sanmee R, Lumyong P, Lumyong S. (2006) The sequestrate genus Rhodactina (Basidiomycota, Boletales) in northern Thailand. Mycotaxon 96: 133–140. [Google Scholar]
- Zhao K, Wu G, Feng B, Yang ZL. (2014a) Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycological Progress 13: 1127–1136. https://doi.org/10.1007/s11557-014-1001-3 [Google Scholar]
- Zhao K, Wu G, Yang ZL. (2014b) A new genus, Rubroboletus, to accommodate Bonicus and its allies. Phytotaxa 188: 61–77. https://doi.org/10.11646/phytotaxa.188.2.1 [Google Scholar]
- Zhu XT, Wu G, Zhao K, Halling RE, Yang ZL. (2015) Hourangia, a new genus of Boletaceae to accommodate Xerocomus cheoi and its allied species. Mycological Progress 14: 37. https://doi.org/10.1007/s11557-015-1060-0
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.