Abstract
The fundamental unit of rapid, physiological color change in vertebrates is the dermal chromatophore unit. This unit, comprised of cellular associations between different chromatophore types, is relatively conserved across the fish, amphibian, and reptilian species capable of physiological color change and numerous attempts have been made to understand the nature of the four major chromatophore types (melanophores, erythrophores, xanthophores, and iridophores) and their biochemical regulation. In this review, we attempt to describe the current state of knowledge regarding what classifies a pigment cell as a dynamic chromatophore, the unique characteristics of each chromatophore type, and how different hormones, neurotransmitters, or other signals direct pigment reorganization in a variety of vertebrate taxa.
Keywords: chromatophore, hormone, neurotransmitter, physiological color change, pigment, vertebrate
Introduction
In the animal kingdom, there are few phenomena as fascinating as the ability to quickly change color and pattern, an ability that can be used to hide from a predator or communicate with a conspecific. Rapid color change on the scale of seconds to hours, termed physiological color change, is typically accomplished via mobilization of pigments or nanostructures within specialized cells called chromatophores (Bagnara and Hadley 1973; Aspengren et al. 2009). Although physiological color change has been observed in insects (Hinton and Jarman 1973), arachnids (Insausti and Casas 2008), crustaceans (Brown and Sandeen 1948), and cephalopods (Florey 1969; Hanlon and Messenger 1988; Messenger 2001), the structures and mechanisms of invertebrate color change are markedly different than those of vertebrates (Umbers et al. 2014). As such, this review focuses only on color-changing vertebrates (not including the birds and mammals which change color by altering blood flow to exposed skin; Rhodes et al. 1997) so that we might better describe the biochemical make-up and regulation of the motile chromatophores responsible for physiological color change in these taxa. Specifically, the objective of our review is to provide a detailed examination and description of the signaling molecules that influence the different types of chromatophore associated with physiological color change in vertebrates, complementing a recently published summary of several of these mechanisms (Sköld et al. 2013). We focus primarily on proximate mechanisms (i.e., biochemistry and cellular mechanisms) of rapid pigment translocation, and largely ignore the ultimate functions (i.e., the selective benefits that physiological color change confers) of physiological color change. Why physiological color change has evolved multiple times within vertebrates and how physiological color change confers selective advantages are equally interesting topics for discussion, but will not be addressed in detail.
Chromatophores are colorful cells that can be classified loosely by their overall color but are more accurately designated by their color-producing mechanisms or the types of pigments/structures that they contain (Aspengren et al. 2009). Pigmentary chromatophores impart color by selectively absorbing particular wavelengths of lights, whereas structural chromatophores produce color by reflecting and scattering particular wavelengths of light with cellular nanostructures. Among the pigmentary chromatophores that impart color via absorption of specific wavelengths of light, there are three predominant chromatophore types found in color-changing vertebrates: the black-brown melanophores (which contain melanin), red erythrophores, and yellow xanthophores (both of which can contain either carotenoids, pteridines, or some combination of the two). Additionally, there are two structural chromatophore types that imbue organisms with color via reflectance of light: the colorless iridophores and the white light-reflecting leucophores (both containing purines; Bagnara and Hadley 1973; Bagnara and Matsumoto 2006; Aspengren et al. 2009). All integumentary vertebrate chromatophore types originate from the embryonic neural crest (Bagnara and Hadley 1973; Fujii 1993; Bagnara and Matsumoto 2006), a migratory, multipotent cell population (Le Douarin and Kalcheim 1999). As chromatophores mature, they typically form functional associations with each other, termed “dermal chromatophore units”, just below the epidermis (Bagnara et al. 1968; Bagnara and Hadley 1973).
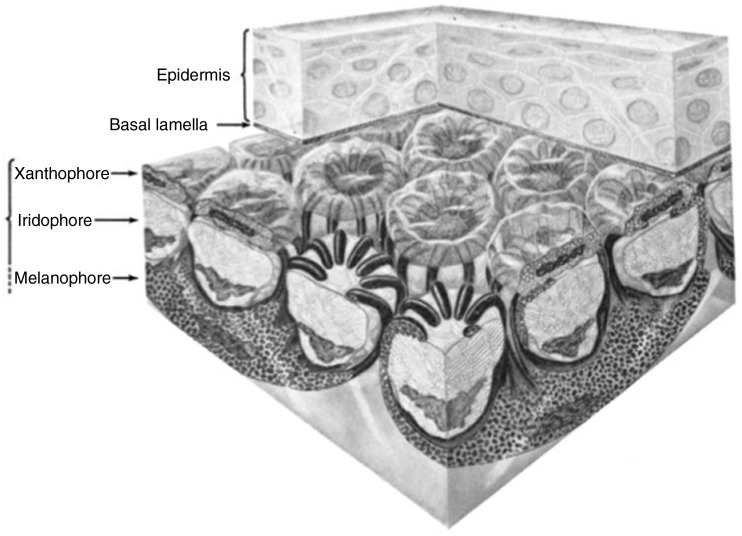
Considered the fundamental unit of physiological color change in vertebrates, the dermal chromatophore unit incorporates melanophores, erythrophores, xanthophores, and iridophores into an isolated cellular system (Bagnara et al. 1968; Bagnara and Hadley 1973; Figure 1). This functional unit is capable of producing a wide range of colors by absorbing or reflecting specific wavelengths of light (Bagnara and Hadley 1973; Grether et al. 2004). From surface of the integument to the base of the dermis, the chromatophore unit consists of the dendritic processes of the melanophore, a single layer of xantho- or erythrophores, a layer of iridophores, and the main cell body of the melanophore (Bagnara et al. 1968; Bagnara and Hadley 1973; Figure 1). It should be noted that, as conserved as this functional unit might be, the relative amounts of each chromatophore type and their relative arrangements vary from species to species and can even vary from one area to another on the same animal (Bagnara and Hadley 1973).
Figure 1.
Schematic representation of the dermal chromatophore unit, shown in the dispersed state.
Notes: The dendritic processes of the basal melanophores, extending around and over the iridophores layer, are filled with melanosomes. Above the iridophores and the melanophore processes is the xanthophore (or erythrophore layer). Figure reproduced from Bagnara et al. (1968) under a Creative Commons License.
While the dermal chromatophore unit can be considered to function as a single element with respect to color generation, the actual color reflected is the result of the four individual types of chromatophores serving different roles by undergoing dynamic, coordinated pigment reorganization (Bagnara et al. 1968; Bagnara and Hadley 1973; Cooper and Greenberg 1992; Grether et al. 2004; Aspengren et al. 2009). In general, the red-yellow erythrophores (or xanthophores) absorb the shorter wavelengths of light incident upon the dermal chromatophore unit, and the remaining wavelengths are either reflected back by the silvery iridophores or absorbed by the brown-black melanophores (Bagnara et al. 1968; Bagnara and Hadley 1973; Nielsen 1978; Aspengren et al. 2009; Figure 2). It is the relative state of pigment dispersion and aggregation in each chromatophore, as well as their relative densities, that determines how much each contributes to the observed color of skin (Bagnara et al. 1968; Grether et al. 2004).
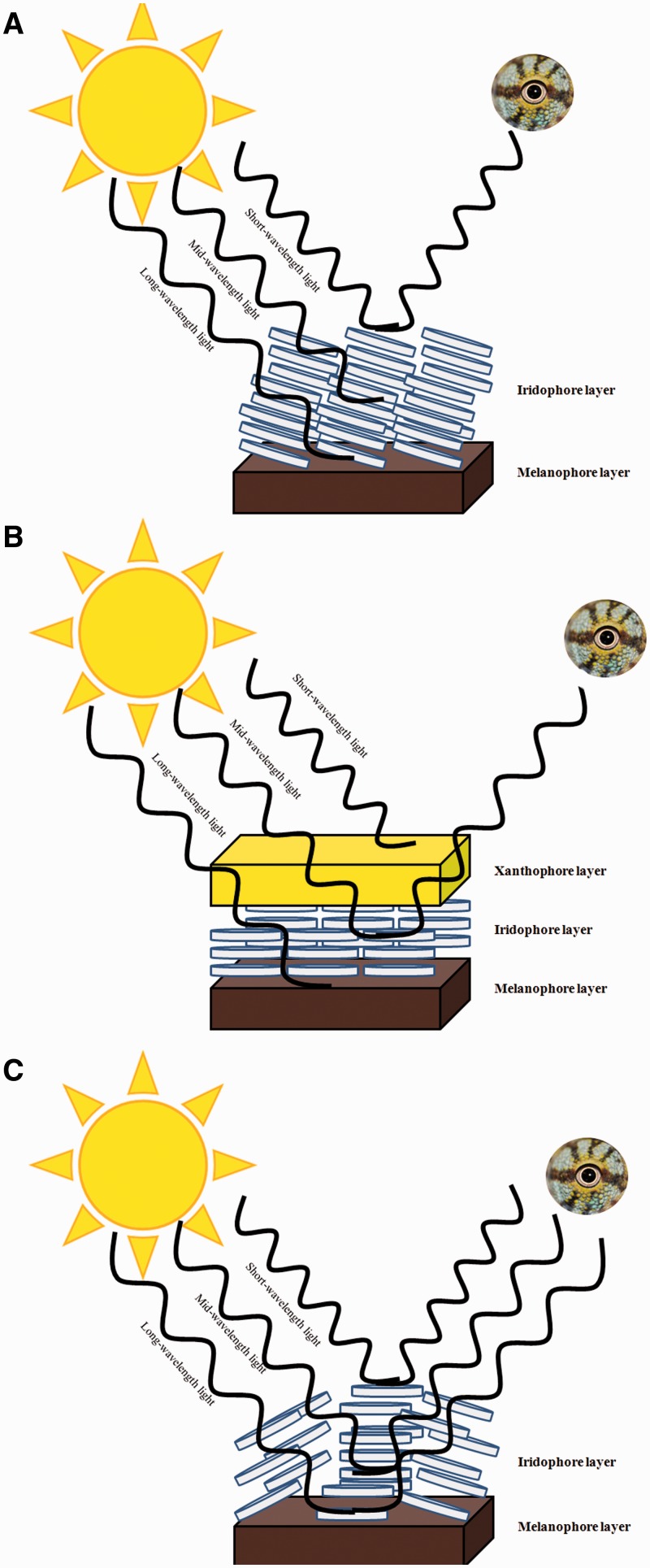
Figure 2.
Schematic representation of the potential contributions of the different elements of the dermal chromatophore unit to reflected light (i.e., color and brightness). Light from an external source (e.g., the sun) interacts with the dermal chromatophore, which influences the wavelengths of light transmitted from the body (and which become visible to external viewers).
Notes: (A) Blue color can be attained when reflecting platelets in the iridophore layer selectively reflect short wavelengths of light and longer wavelengths of light are absorbed by the melanin-containing melanophore layer. (B) Green or yellowish-green is reflected when the xanthophore layer absorbs short-wavelengths of light, the platelets in the iridophore layer reflect medium wavelengths of light, and the melanophore absorbs longer wavelengths of light. (C) White light is reflected when iridophore platelets reflect or scatter all incoming wavelengths of light. Figure after Bagnara and Hadley (1973).
It is generally accepted that pigment mobilization must be carried out by motor proteins, such as kinesin, dynein, and myosin, which move along tracks provided by structural elements of the cytoskeleton (Bikle et al. 1966; Rodionov et al. 1991; Nilsson and Wallin 1997; Tuma and Gelfand 1999; Aspengren et al. 2009). A model pathway proposed by Nery and Castrucci in their 1997 review describes the general pattern between receptor activation and pigment granule dispersal: First, a rise in intracellular second messenger (e.g., cyclic adenosine monophosphate = cAMP) concentration is induced by the binding of a signal molecule to its receptor on the plasma membrane (Nery and Castrucci 1997). Next, the higher levels of cAMP activate protein kinase-A, which phosphorylates proteins bound to the pigment granules, causing these proteins to detach from the cytoskeleton and allowing the (recently freed) granule to be transported by kinesin (Figure 3A) away from the perinuclear area (Nery and Castrucci 1997). Conversely, pigment aggregation is typically achieved when protein kinase-A is inactivated, leading to protein dephosphorylation, which enables dynein to transport pigments back toward the center of the cell (Figure 3B). This model, while simplified, allows for the generalization that extracellular signals can results in an intracellular cascade that causes pigment granules to associate with motor proteins to initiate color change.
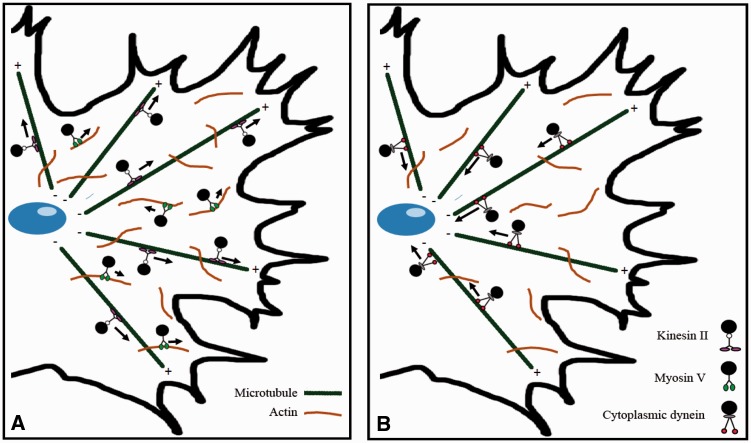
Figure 3.
Model of melanosome transport during dispersion and aggregation, redrawn from Tuma and Gelfand (1999).
Notes: (A) Long-range melanosome dispersion occurs via transport by kinesin along microtubules. After reaching the cellular periphery, melanosomes are transported along randomly oriented actin filaments by myosin motor proteins. (B) Melanosome aggregation occurs via microtubules but, in contrast to pigmentary dispersion, takes place with the aid of dynein molecular motors.
Across vertebrate taxa, the greatest differences in the regulation of physiological color change depend on whether the integument is under neural control, endocrine control, or some combination of the two (Nery and Castrucci 1997). However, given the broad range of color combinations that can be created by the dermal chromatophore unit (Grether et al. 2004), an appreciation of the complex regulatory pathways of each chromatophore type is essential for understanding both inter- and intraspecific differences in chromatophore control. In this review, we examine the effects of hormones, neurotransmitters, intracellular second messengers, and even ambient light on the chromatophores associated with physiological color change.
Melanophores
Content and structure
The melanophore’s appearance is affected by the organization of its melanosomes (or melanin granules), which are vesicular organelles that exclusively contain melanin (Bagnara and Hadley 1973). “Melanin” is a general term for all members of the tyrosine-derived class of pigments found in melanophores, which have high molecular weights and are chemically stable (Lerner and Fitzpatrick 1950; Nicolaus and Piattelli 1962; Ito and Wakamatsu 2003). While all melanins exhibit these properties, there are several sub-classifications of melanin: neuromelanin, allomelanin, pheomelanin, and eumelanin (Fedorow et al. 2005). Neuromelanin is specifically expressed in the central nervous systems of primates (Marsden 1961; Fedorow et al. 2005), while allomelanin is found in the fungi, plant, and bacteria kingdoms (Fedorow et al. 2005). Pheomelanin ranges from a muted yellow to red and is traditionally been recognized only in mammalian and avian melanocytes (Ito and Wakamatsu 2003), though it has also recently been discovered in the shell of Hermann’s tortoises Eurotestudo boettgeri (Roulin et al. 2013). Finally, there is the brown-black eumelanin, or “true melanin,” that is produced by birds, mammals, and all vertebrates capable of physiological color change (Aspengren et al. 2009) and this is the only melanin to which we will refer in this review.
Melanophores are capable of synthesizing their own melanin (Seiji et al. 1961, 1963) and, based on the final destination of the melanin produced, can be classified as either epidermal or dermal. Epidermal melanophores, responsible for the slower process of morphological color change, are commonly described as “spindle-shaped” with varying degrees of dendritic branching and are positioned at the base of the epidermis (Bagnara and Hadley 1973). The cell body of the epidermal melanophore produces melanin granules and the processes, which weave around adjacent epidermal cells, are the sites of deposition of these granules into other cells (Bagnara and Hadley 1973). Dermal melanophores, often associated with the dermal chromatophore unit, are found in the dermis and consist of a central cell body with dendritic processes that can be directed radially or upward toward the epidermis, giving the cell a basket-like shape (Bagnara and Hadley 1973; Nielsen 1978). Dermal melanophores retain all synthesized melanin and are, unlike the other types of chromatophores, exclusive to vertebrates capable of physiological color change (Bagnara and Hadley 1973).
Given that color change takes time, even for organisms capable of very rapid color change, there was once a health debate about whether the dendritic melanophore processes were motile projections that simply carried melanosomes with them, or if the processes were static and only melanosomes translocated within them (Bagnara et al. 1968). However, Bagnara et al. (1968) observed that melanophores in the aggregated state (i.e., melanosomes clustered in the main cell body) possessed membranous layers where once melanophore processes had existed in the dispersed state. This evidence indicated that, in the absence of melanosomes, the processes had simply been emptied and collapsed in place rather than being drawn back into the cell body of the melanophore (Bagnara et al. 1968). Further support for the claim that melanophore processes are permanent structures is that the interactions between iridophore and melanophore plasma membranes are very intimate (Bagnara et al. 1968). For example, even when the melanophore is in the aggregated state, there are non-random indentations in the iridophore plasma membrane corresponding to the locations of known melanophore processes when dispersed (Bagnara et al. 1968). From this seminal work, the primary mechanism of physiological color change was determined to be the result of dynamic intracellular pigment transportation.
Mechanisms of color change
Although the specific factors that regulate melanosome motility frequently differ among taxa (see below), the mechanics of pigment translocation within melanophores appear to be relatively conserved among different groups. Exactly how are melanin granules transported so quickly within the melanophore, especially given their rather large size (up to several hundred microns in diameter; Bagnara and Hadley 1973)? The process of melanosome translocation is thought to be coordinated by the microtubule (MT) and actin filament architecture of the cytoskeleton and the associated motors (Bikle et al. 1966; Rodionov et al. 1991, 2003; Nilsson and Wallin 1997; Tuma and Gelfand 1999; Figure 3). Specifically, the molecular motor kinesin transports melanin granules toward the cell periphery during pigment dispersal (Rodionov et al. 1991), where melanosomes are subsequently transported along randomly oriented actin myofilaments (Tuma and Gelfand 1999; Rodionov et al. 2003; Figure 3A). When melanophores receive input producing melanosome aggregation, the tubulin-based molecular motor dynein hauls melanin granules back toward the nucleus (Nilsson and Wallin 1997; Figure 3B). For example, Logan et al. (2006) showed that MTs are essential for melanosome transport in zebrafish Danio rerio by administering MT disrupting agents and observing that transport was diminished, slowed, or eliminated. These authors also investigated the role of actin-based microfilaments and found that they may have a role in tethering melanosomes to the periphery of the cell after dispersion. This “tethering” could be a mechanism to reduce the cost of color change, by reducing the amount of cellular signalling and energy currency spent to keep the melanophores stellate.
Influence on color
In color changing vertebrates, the distribution of melanosomes within dermal melanophores (hereafter “melanophores”), especially in their dendritic processes, is a primary determinant of the lightness of the animal’s skin color. That is, as the area of melanosome distribution increases, more light is absorbed and a darker color is produced. Interestingly, however, melanophore aggregation and dispersal do not appear to be dose-dependent processes in the zebrafish D. rerio. Instead, the melanophores of this species consistently demonstrate a translocation threshold where they completely aggregate or completely disperse if at least the minimum dose of translocation agonist necessary to stimulate pigment migration is administered (Logan et al. 2006). Hence, variation in the extent of dispersal would refer primarily to the rate of dispersal in this species, as the pigment granules move through the intermediate stages of translocation from one extreme to the other (Logan et al. 2006). The binary nature of pigment dispersion observed in zebrafish may be species-specific, however, and more work is required to better understand how broadly this phenomenon extends among color changing vertebrates.
Regulation of melanosome motility within melanophores
The discovery of motile melanosomes triggered an abundance of investigations into the mechanisms and regulation of melanosome transport within melanophores, especially in species capable of rapid and possibly facultative color change (see reviews in Bagnara and Hadley 1973; Nery and Castrucci 1997; Aspengren et al. 2009; Stuart-Fox and Moussalli 2009; Nilsson Sköld et al. 2013). Rapid, facultative color change has since been studied in a wide variety of vertebrate species including fish, amphibians, and reptiles (Wright and Lerner 1960; Bagnara and Hadley 1973; Nielsen 1978; Hadley et al. 1985; Okelo 1986; Maeno and Iga 1992; Summers and Greenberg 1994; Kotz and McNiven 1994; Osorio and Vorobyev, 1996; Larson and Summers 2001; Mäthger et al. 2003; Logan et al. 2006). For melanophores, cAMP appears to be one of the most prevalent and consistently agreed-upon cellular regulators of melanosome dispersal (reviewed in Tuma and Gelfand 1999; see also Fujii 2000). Numerous studies have found that an increase in the intracellular concentration of cAMP is associated with a dispersed melanosome distribution, whereas a decrease in cAMP causes the melanophore to reassume the aggregated state (Rozdzial and Haimo 1986; Sammak et al. 1992; Fujii 2000; Logan et al. 2006). In an experiment by Maeno and Iga (1992), forskolin, a potent activator of the cAMP synthesizing enzyme adenylate cyclase, was administered to melanophores and proved to be a powerful dispersant as well as an effective blocker of induced aggregation. Alpha-melanophore-stimulating hormone (α-MSH) and adrenocorticotropic hormone, collectively referred to as melanocortins, are also known to activate both the cAMP synthesizing enzyme adenylate cyclase and stimulate melanosome dispersal in reptiles, amphibians, and fish (Hadley and Goldman 1969; Goldman and Hadley 1970; Okelo 1986; Maeno and Iga 1992; Fujii 2000).
Agouti proteins also function in color change processes by antagonizing melanocortin receptors and, consequently, counteracting the effects of α-MSH (Cerdá-Reverter et al. 2005; Zhang et al. 2010). Increased levels of agouti signaling protein are also associated with lighter colors and fewer melanosomes (Guillot et al. 2012). Up-regulation of specific agouti proteins even appears to underlie the background matching observed in zebrafish (Zhang et al. 2010), preventing α-MSH from elevating intracellular levels of cAMP. In addition to the effects of α-MSH and cAMP on color change, many studies provide evidence that calcium ions (Ca2+) play an important role in physiological color change via their role as chemical messengers (Novales and Novales 1965; Van De Veerdonk and Brouwer 1973; de Graan et al. 1982a, 1982b; Iga and Takabatake 1982). Despite these empirical results to the contrary, Kotz and McNiven (1994) assert that there is no evidence indicating a link between Ca2+ concentrations and melanosome motility. Therefore, it seems that the involvement of calcium ions in the regulation of pigment responses is still somewhat controversial (reviewed in Nery and Castrucci 1997).
While α-MSH is considered a pigment dispersant across all taxa, two other hormones are associated with pigment cell motility: melatonin and melanin-concentrating hormone (MCH). These hormones can behave as either dispersants or aggregators at different concentrations in the melanophores of different species (Fujii 2000; Aspengren et al. 2009). Melatonin is generally considered an aggregation inducer in fish, though melatonin sensitivity is highly variable among species (Nery and Castrucci 1997; Fujii 2000). While it has been suggested melatonin indirectly inhibits adenylate cyclase (Filadelfi and Castrucci 1996), supporting its usual role as an aggregation agent (Rollag 1988; Sugden 1991; Fujii 1993), melatonin can actually elicit pigment dispersion in some fish species (reviewed in Fujii 2000) or even promote opposite responses in different areas of the same fish’s skin (Nishi and Fujii 1992). Similarly for MCH, which is typically an aggregator (Oshima et al. 1986; Fujii 2000; Logan et al. 2006; Aspengren et al. 2009), studies have found that high doses of MCH can induce dispersion (within teleosts). This duality of effect led to the hypothesis that there are two different types of MCH receptors with either high or low MCH affinity (Oshima et al. 2001; Aspengren et al. 2009). Reptiles and amphibians are much less sensitive to MCH but, if given in high concentrations, MCH can stimulate melanosome dispersal (Wilkes et al. 1984; Fujii 2000).
Primitive fishes are most susceptible to the influence of the hormones α-MSH, MCH, and melatonin because, unlike the bony teleost fishes, their physiological color change is mostly controlled by the endocrine system (Fujii 2000). Physiological color change in teleost fish is also controlled by sympathetic enervation, making them sensitive to the catecholamine neurotransmitters epinephrine (EPI) and norepinephrine (Fujii and Oshima 1986; Maeno and Iga 1992; Fujii 2000). The sympathetic nervous system is responsible for the flight-or-fight response to stressful stimuli, which is brought about by the release of catecholamines that can act on either α- or β-adrenoceptors (Maeno and Iga 1992). The roles of α- and β-adrenoceptors are conserved across all fish, reptile, and amphibian taxa, and these two types of receptors are often found within the same organism, either restricted locally or commingling in the plasma membrane of the same melanophore (Vaughan and Greenberg 1987; Summers and Greenberg 1994). Stimulation of α-adrenoceptors initiates a decrease in intracellular levels of cAMP concomitant with an aggregation response, while catecholamines can simultaneously activate β-adrenoceptors, which induce an increase in cAMP and causes melanosome dispersal (Maeno and Iga 1992; Kotz and McNiven 1994; Nery and Castrucci 1997; Fujii 2000).
Competition between α- and β-adrenoceptors was first demonstrated in the green anole Anolis carolinensis by Vaughan and Greenberg in 1987. Because sympathetic nerves do not innervate anole skin, all physiological color change is mediated by circulating catecholamines binding to adrenoceptors and their respective effects on adenylate cyclase activity (Vaughan and Greenberg 1987). These authors found that the eyespots of the green anole lack α-adrenoceptors, in contrast to the rest of the anole dermis (Vaughan and Greenberg 1987). All other anole melanophores have α- and β-adrenoceptors, which are both stimulated by EPI (Vaughan and Greenberg 1987). The β-receptors of the melanophores are activated by low levels of EPI and result in skin darkening, whereas high levels of EPI stimulate the α-adrenoceptors of those same melanophores to override the β-adrenoceptor response by inhibiting adenylate cyclase and thus the skin lightens (Vaughan and Greenberg 1987). Therefore, an anole under duress will appear green everywhere except for its eyespots, which will be dark brown. Vaughan and Greenberg (1987) also found that anoles pretreated with a β-specific adrenergic blocker, propranolol, prior to treatment with EPI exhibited significantly retarded eyespot formation and were unable to attain the maximal dark brown body coloration. Vaughan and Greenberg (1987) suggest that both of these effects occurred as a result of EPI’s ability to activate only α-adrenoceptors in propranolol-treated animals.
Summary
The melanophore is an important and dynamic contributor to physiological color change in fish, amphibians, and reptiles. There has been a great deal of research on the effects of particular compounds, hormones, and neurotransmitters (e.g., α-MSH, MCH, melatonin, catecholamines, and cAMP) on the translocation of the melanin granules from the perinuclear area of the melanophore cell body into the dendritic processes. Most of the aforementioned compounds act on receptors in the melanophore cell membrane and elicit a change in the activity of adenylate cyclase, and therefore a change in the intracellular concentration of cAMP. However, several classes of receptor are frequently present on individual melanophores, suggesting that there is a great deal of complexity in the biochemical regulation of melanosome organization and additional research remains to be done before we can fully clarify the range of interactions between different receptor types and adenylate cyclase. Furthermore, considering the controversy surrounding the role of Ca2+, its relationship to melanophore motility in fish, amphibians, and reptiles should be more specifically investigated. Additionally, the melanin pigment is ubiquitous across many more taxa than those discussed here, and further research has the potential to discover and clarify the additional functions it can serve in biological systems. Moving beyond melanophore-specific examinations, in-depth investigations of the dynamic interactions between melanophores and the other chromatophore types, which produce the remarkable color displays of fish, amphibians, and reptiles capable of physiological color change, should be a particularly fruitful area of future research.
Erythrophores and Xanthophores
Content, structure, and color
Erythrophores and xanthophores are two additional chromatophore types associated with the dermal chromatophore unit in vertebrates capable of physiological color change. These chromatophores are loosely classified by their predominant coloration, with xanthophores being principally yellow and erythrophores being principally red (Bagnara and Hadley 1973). For some physiologically color changing vertebrates, including a variety of fish (Satake 1980; Hadley et al. 1985; Oshima et al. 1986; Kotz and McNiven 1994; Sato et al. 2004) and amphibians (Bagnara et al. 1968; Nielsen 1978), it has been determined that erythrophores and xanthophores are dynamic contributors to color change. However, there is no evidence that this is the case with all color changing vertebrates; there may be some species where the erythrophores or xanthophores serve only a passive role, and melano- and iridophores are the only dynamic chromatophores. For example, there is currently no definitive evidence of a dynamic contribution of the erythrophore/xanthophores to the rapid color change of any reptile species.
The yellow-to-red coloration of xanthophores and erythrophores is the result of the relative amounts, and types, of pteridine and carotenoid pigments within each chromatophore (Bagnara and Hadley 1973). Traditionally, there are thought to be only two major pteridines involved in color change, drosopterin and sepiapterin (Obika et al. 1964), though drosopterin can exist in three forms (drosopterin, isodrosopterin, and neodrosopterin, all of which are found in the lizard Sceloporus virgatus; Weiss et al. 2012) and xanthopterin may underlie some orange skin coloration in reptiles and amphibians (Suga and Munesada 1988; Morrison et al. 1995). In comparison, there are two entire families of carotenoids involved in physiological color change: the hydrocarbon carotenes and the oxygen-containing xanthophylls (Bagnara and Hadley 1973). However, Chatzifotis et al. (2011) recognize two different classes of carotenoids based primarily on the color these molecules reflect: the yellows (e.g., beta-carotene, lutein, zeaxanthin) and the reds (e.g., astaxanthin esters). Because the wavelengths of light absorbed by carotenoid molecules (and hence, the wavelengths of light reflected which provide color) are dependent on the length of their conjugated pi-systems (Allen 2008) and not the presence or absence of oxygen, the carotenoid classification suggested by Chatzifotis et al. (2011) represents a fundamentally different criterion for grouping carotenoid pigments.
The diversity of carotenoid types present in erythrophores and xanthophores is largely due to the fact that carotenoids can only be obtained through an animal’s diet, as opposed to pteridines which can be synthesized de novo within the chromatophores (Bagnara and Hadley 1973). Carotenoids are ingested rather indiscriminately, as different types of carotenoids often coexist within the same food source, and it is only after ingestion that animals can modify and metabolically convert the carotenoids according to established biochemical pathways (reviewed in Møller et al. 2000; McGraw 2006; Svensson and Wong 2011). On the other hand, only drosopterin (red) and sepiapterin (yellow) can be produced in abundance de novo in the vertebrate taxa examined in this review (Hama 1963; Obika 1963; Bagnara and Hadley 1973; Ichikawa et al. 1998). While the sepiapterin synthesis pathway has not yet been fully elucidated, it is known that once sepiapterin is formed from guanosine triphosphate, it often serves as a precursor of further pteridine differentiation (reviewed in Ziegler 2003). However, sepiapterin is not a precursor to drosopterin; rather there is a branching point in the biochemical pathway from which both sepiapterin and drosopterin are synthesized from the same intermediate (Dorsett et al. 1979). Given the shared pathways of pteridine synthesis, it is surprising that individual chromatophores synthesize either drosopterin (erythrophores) or sepiapterin (xanthophores), to the near exclusion of the other type (Ichikawa et al. 1998).
Because xanthophores and erythrophores can both contain carotenoid vesicles, pterinosomes, or some combination of the two, the terms xanthophore and erythrophore are often used interchangeably. A commonly used subjective distinction between the two relies simply on whether the chromatophore appears more yellow (xanthophore) or red (erythrophore; Bagnara and Hadley 1973). Despite their overall similarity, xanthophores and erythrophores are distinct entities with subtle, but significant differences. One of these differences, exclusivity of pteridine production between chromatophore types (Ichikawa et al. 1998), has already been discussed. Other differences are related to the development and maturation of the chromatophores themselves. In a study of the metamorphosing brown frogs Rana ornativentris, Ichikawa et al. (1998) found that erythrophores were first observed in the hypodermis several stages after the first of the already formed xanthophores were finally moving out of the collagenous layer, where they were formed just above the hypodermis, into the subepidermal space that is the residence of mature dermal chromatophores (Ichikawa et al. 1998). As metamorphosis progressed, the xantho-, irido-, and melanophores assumed the arrangement of the typical dermal chromatophore unit, and were eventually joined by the erythrophores, which formed an unusual extra layer below the chromatophore unit (Ichikawa et al. 1998). This morphological evidence indicates that erythrophores and xanthophores originate independently of each other, at least in R. ornativentris.
In R. ornativentris, the major difference between xanthophores and erythrophores with regard to the carotenoid vesicles is in their respective quantities and distributions (Ichikawa et al. 1998). The carotenoid vesicles of erythrophores are generally restricted to the cytoplasmic periphery, whereas the more numerous carotenoid vesicles of xanthophores are found both near the nucleus and dispersed in the cytoplasm (Ichikawa et al. 1998). While the carotenoid vesicles were very similar in structure and development in the two chromatophores, there were stark differences in their pterinosome ontogeny (Ichikawa et al. 1998). In the xanthophore, development of the fibrous, concentric whorl (a characteristic property of pterinosomes) began at the center of the granule, whereas the whorl developed first at the periphery of erythrophore pterinosomes (Ichikawa et al. 1998). Also, pterinosomes in the erythrophore remained uniform in size throughout metamorphosis and on into the juvenile frog stages, whereas those of the xanthophore were diverse and grew in size during metamorphosis, but then shrunk when the frog completed the transition from tadpole to juvenile (Ichikawa et al. 1998). It is noteworthy to point out that, in addition to the pterinosomes of the respective chromatophores being significantly different in size, these two distinct types of pterinosomes were never observed to coexist within one chromatophore (Ichikawa et al. 1998). Given that this study was conducted on a single species, the general applicability of these conclusions is not known.
Mechanisms of color change
The mechanism of pigment granule reorganization is believed to be similar, if not the same, in erythrophores and melanophores in the sense that pigment granules in both cell-types associate with motor proteins for transport (Byers and Porter 1977; Kotz and McNiven 1994; Nery and Castrucci 1997). However, in contrast to melanophores, both the aggregation and dispersion processes in erythrophores were approximately twice as fast as melanosome translocation in melanophores (Kotz and McNiven 1994). Furthermore, erythrophore pigments appear to aggregate quickly and with uniform velocity, while dispersing with saltatory motions at a slower rate (Byers and Porter 1977; Beckerle and Porter 1983; Kotz and McNiven 1994). The difference in dispersal and aggregation behavior of erythrophore pigments is likely due to the structural changes in the erythrophore during these different stages (Byers and Porter 1977). Specifically, pigment cells aggregate by moving along microtubules within a three-dimensional lattice that is also withdrawn during aggregation. The restructuring of the collapsed lattice, required for pigments to be subsequently translocated away from the nucleus (i.e., dispersed), may account for the different rates of pigment movement between aggregation and dispersal (Byers and Porter 1977). Additionally, the apparent differences in pigment granule motility between melanophores and erythrophores led Beckerle and Porter (1983) to investigate the effects of MT disruption and actin motility inhibition on pigment motility within erythrophores in squirrelfish Holocentrus adscensionis chromatophores. The MT depolymerizing agents colchicine and nocodazole were used to remove the radial MT cytoskeletal network, beginning at the cell periphery and depolymerizing toward the nucleus (Beckerle and Porter 1983). In one treatment, the MTs were partially depolymerized while the pigment granules were retained in a dispersed state by administration of caffeine; after a sufficient amount of depolymerization had been allowed, EPI was given to induce aggregation (Beckerle and Porter 1983). Aggregation only occurred where MTs persisted closer to the nucleus and any pigment left farther out than the MTs extended remained dispersed (Beckerle and Porter 1983). In a second experiment, MTs were completely depolymerized with nocodazole, and pigment granules lost their characteristic radial array and subsequent administration of EPI only induced localized clumping of pigment granules (Beckerle and Porter 1983). Therefore, Beckerle and Porter (1983) concluded MTs are required for organized pigment translocation. In addition, an actin inhibitor, cytochalasin B, was administered to erythrophores, but no change to pigment motility was observed, which indicates that pigment dispersion and aggregation in erythrophores is independent of actin, unlike melanophores (Beckerle and Porter 1983).
Regulation of pigment motility
In a parallel to melanophore responses to intracellular cAMP levels, calcium ions are the predominant signal for pigment aggregation and dispersion in erythrophores (Kotz and McNiven 1994). Kotz and McNiven (1994) investigated the effect of intracellular calcium ion concentrations (Ca2+) in the intact, un-lysed erythrophores of squirrelfish H. adscensionis and found that an increase in Ca2+ was a concomitant, sufficient, and necessary requirement for pigment aggregation (Kotz and McNiven 1994). Interestingly, while the rise in Ca2+ is required for aggregation, the pigments remain aggregated even after Ca2+ has returned to basal levels (Kotz and McNiven 1994). This phenomenon led Kotz and McNiven (1994) to the conclusion that there must be another signaling molecule involved in pigment translocation and they turned their attention to the effect of changing cAMP levels on erythrophores, due to the importance of cAMP in melanophore motility. They found that a decrease in Ca2+ was necessary for pigment dispersion, but dispersal did not occur unless there was also simultaneous increase in cAMP (Kotz and McNiven 1994). While this response is analogous to cAMP’s role in melanosome dispersion, a high concentration of cAMP in erythrophores can apparently be overridden by a sufficient increase in Ca2+. In contrast, melanophore motility was completely unaffected by the absence or presence of intracellular Ca2+ (Kotz and McNiven 1994).
Under most conditions, erythrophores (hereafter used to describe both erythrophores and xanthophores, unless xanthophores were specifically studied) respond to extracellular signals with pigment migration patterns similar to the melanophore responses previously described (Oshima et al. 1986; Kotz and McNiven 1994). Erythrophores respond to α-MSH administration with pigment dispersion in teleosts and frogs (Hadley et al. 1985; Oshima et al. 1986). In research conducted by Hadley et al. (1985), a super-potent analog of α-MSH, [Nle4, D-Phe7]-α-MSH or MSH*, was analyzed alongside α-MSH in the teleost Lebistes reticulatus, five species of amphibians (genera Rana, Xenopus, Bufo), the lizard A. carolinensis, and a rattlesnake Crotalus atrox. The analog induced prolonged darkening in all species and, specifically, dispersion in the teleost erythrophores that lasted 6 days after saline rinse compared with the relatively fast re-aggregation time of 4 h for melanophores (Hadley et al. 1985). These results indicate that not only is α-MSH able to directly act upon erythrophores in addition to melanophores, there also appears to be significantly different mechanism or potency of interaction between the hormone and the two chromatophores (Hadley et al. 1985).
As in melanophores, the hormones melatonin and MCH also induce pigment migration in erythrophores (Satake 1980; Oshima et al. 1986); however, the catecholamine neurotransmitters EPI and norepinephrine have a more direct, one-sided relationship with erythrophores than melanophores (Beckerle and Porter 1983; Oshima et al. 1986). Satake (1980) used melatonin as a control aggregation agent in the xanthophores of the common goldfish Carassius auratus when testing the effects and interactions of novel dispersants; thus, melatonin’s role has so far only been observed to be an aggregation agonist in the xanthophores of fish species, as opposed to its bidirectional, dose-dependent influence in fish melanophores (reviewed in Fujii 2000). Similarly, MCH also exclusively causes aggregation in both erythrophores and xanthophores of several teleost species (Oshima et al. 1986). While MCH and α-MSH are considered antagonizing hormones, MCH can induce aggregation in the absence of Ca2+, unlike the Ca2+-dependent dispersion agent, α-MSH (Oshima et al. 1986). Oshima et al. (1986) also tried to determine whether the effects of MCH are transduced by α- or β-adrenoceptors by administering MCH after treatment with either an α- or β-adrenoceptor blocking agent. Although erythrophore pigment motility induced by MCH was not found to make use of either adrenoceptor, the neurotransmitters EPI and norepinephrine certainly do (Oshima et al. 1986). As in melanophores, stimulation of α-adrenoceptors by catecholamines caused pigment aggregation in erythrophores (Oshima et al. 1986). However, despite the coincidence of α- and β-adrenoceptors on the erythrophores, there was no mention of catecholamines stimulating β-adrenoceptors and causing pigment dispersal (Oshima et al. 1986). Thus, information regarding the effects of β-adrenoceptor stimulation of erythrophores in teleosts is lacking. There is also a scarcity of research regarding the effects of melatonin, MCH, or catecholamines on the erythrophores of reptiles and amphibians; however, this is most likely due to the once prevalent belief that erythrophores only passively contributed to physiological color change in these taxa, acting as static filters to remove particular wavelengths of light (Bagnara and Hadley 1973). Although light filtering is probably a primary function of erythrophores, these chromatophores also appear to provide dynamicity of physiological color change in some organisms by varying the extent of filtration (Satake 1980; Hadley et al. 1985; Oshima et al. 1986; Kotz and McNiven 1994).
The dynamic filtration interaction between ambient light and erythrophores is perhaps best demonstrated not by chemical induction, but through pigment motility in response to different wavelengths of available light. In natural conditions, erythrophores are exposed to both the full spectrum of visible light and ultraviolet (UV) radiation; however, the results from a study by Sato et al. (2004) showed that exposure to different wavelengths of light, ranging from 365 (UV) to 600 nm (red end of visible spectrum), affected the direction of pigment motility in the Nile tilapia Oreochromis niloticus. Specifically, Sato et al. (2004) found that erythrophores dispersed their pigment at 440–550 nm (blue and green light) and aggregated their pigments at all other wavelengths (365–440 nm, 550–600 nm) as well as in darkness (Sato et al. 2004). These results make sense because the pigments of erythrophores (and xanthophores) absorb in the blue and green regions of the spectrum (Allen 2008). The pigments within erythrophores disperse under these conditions because that is when they are capable of maximum absorption, and could provide a protective mechanism to prevent sun damage (Allen 2008).
Summary
Despite reasonably complete knowledge regarding the mechanisms of pigment movement within the erythrophores and xanthophores of color-changing vertebrates, much remains to be uncovered. There is a great deal of support for analogous pigment mobilization in the erythrophores and melanophores of teleosts, yet there has been very little research on the responses of pterinosomes and carotenoid vesicle translocation in the erythrophores of reptiles, amphibians, and other fish species. Given the opposite effects of MCH on fish compared with reptile and amphibian melanophores, it is unknown whether MCH would aggregate pigments in reptile and amphibian erythrophores as it does in fish melanophores, or whether MCH would disperse erythrophore pigments as it does in reptile and amphibian melanophores. Additionally, very little research has been conducted exploring the effects of melatonin on erythrophores of any of the taxa discussed, and more in-depth investigations concerning stimulation of both α- and β-adrenoceptors of erythrophores are certainly needed.
Iridophores (with Brief Discussion of Leucophores)
Iridophores versus leucophores
Iridophores and leucophores are frequently lumped together in a catch-all, “light-reflecting chromatophore” category. Although both provide color by reflecting light (when compared with pigmentary colors which imbue color primarily via selective absorption of particular wavelengths of light), iridophores and leucophores differ from one another in several key ways. First, leucophores reflect only white light, whereas iridophores can reflect specific spectra of light ranging from violet to red, including iridescent coloration (Fujii 1993; Bagnara and Matsumoto 2006). Second, iridophores tend to be located above melanophores within the dermal chromatophore unit (Bagnara and Hadley 1973), while leucophores are typically found below the melanophore layer (Obika 1988). Third, and perhaps most importantly, iridophores and leucophores differ with respect to the way that they affect color change. Leucophores are analogous in both form and function to melanophores in that they posses dendritic processes through which motile sub-units (“leucosomes” or “refractosomes”) travel (Fujii and Miyashita 1979; Obika 1988). In contrast, color change is effected within iridophores by changing the spacing and orientation of orderly distributed, light-reflecting platelets (Bagnara 1966; Taylor 1969; Fujii 1993; see below). Finally, leucophores are known to contribute to natural physiological color change in only a handful of vertebrates, located among the ray-finned fishes (killifish Fundulus (Menter et al. 1979); medaka Oryzias (Obika 1988); guppies Poecilia, formerly Lebistes (Takeuchi 1976)). However, albino individuals of the African clawed frog also possess leucophore-like cells (Xenopus laevis; Fukuzawa 2004). Because leucophores are present only in a restricted subset of color-changing vertebrates, we will focus the remainder of our discussion on the factors influencing color changing iridophores.
Content, structure, and mechanisms of color production
Iridophores, which contain stacks of colorless guanine platelets, effect physiological color change primarily via the alteration of brightness, or relative reflectance, of perceived colors (Bagnara et al. 1968; Bagnara and Hadley 1973; Cooper and Greenberg 1992). The organization of the guanine reflecting platelets into stacks causes iridophores to provide a highly reflective, often iridescent surface in the dermis of the skin (Bagnara et al. 1968; Cooper and Greenberg 1992; Fujii 1993; Nery and Castrucci 1997). Iridophores do not contain an actual “pigment” molecule, rather the mutable arrangement of guanine platelets, in conjunction with their high refractive index, is a dynamic form of structural coloration (Fujii 1993; Prum 2006). Structural colors are produced by the interaction of light with specific, internal or intracellular structures of an organism (Fox 1976; Prum 2006); interactions that result in perceivable visual effects and colors (from metallic or iridescent to yellow or blue) and thus contribute substantially to color changes in the skin (Fujii et al. 1991; Cooper and Greenberg 1992; Morrison et al. 1996).
The color-producing mechanism within iridophores, guanine, is very different from the pigments of melanophores, erythrophores, and xanthophores in two ways: it is inherently transparent and it is also a necessary biochemical component of every living cell (Nelson and Cox 2008). Unlike melanins, pteridines, and carotenoids, which serve, for the most part, an accessory role in pigment cells, guanine is an essential element of DNA and nucleotide-based intracellular energy currency (Nelson and Cox 2008). In addition, there are other molecules that occur in iridophores in trace amounts relative to the abundant guanine, including another essential and ubiquitous purine, adenine, as well as hypoxanthine and uric acid (Stackhouse 1966; Bagnara and Hadley 1973; Ziegler 2003). Although inherently colorless, the tight, but unorganized collection of free guanine sequestered into crystal-like structures imparts a huge refractive index of 1.83 (approximately 75% that of diamond, the most refractive material known) to the iridophore’s reflecting platelets (Land 1972; Fujii et al. 1991; Mäthger et al. 2003; Serway et al. 2009). The organization of these highly refractive reflecting platelets into stacks throughout the cytoplasm, a medium with a refractive index of 1.33, results in the iridophore having a very high reflective capacity (Land 1972; Fujii et al. 1991). For the iridophores involved in physiological color change, this reflectivity is the result of multilayer thin-film interference created by the alternating layers of materials with high (guanine) and low (cytoplasm) refractive indexes (Denton and Land 1971). In addition to differences in the refractive indexes between the guanine and cytoplasm layers, the size, shape, proximity, and orientation of the guanine reflecting platelets influence the observed colors. In most biological systems, including all vertebrate iridophores studied to date, the reflectivity of multi-layer thin-film reflectors is “non-ideal” if the optical thickness of all layers is not uniformly equal (Denton and Land 1971).
Mechanisms of color change
Another distinctive feature of iridophores is that they have been observed to exhibit not one, but two kinds of platelet motion: a sliding, lateral dispersion (similar to dispersion in erythrophores) or an accordion-style stack expansion or compression. Whereas, lateral changes in platelet location have been observed only in gobiid fishes (Iga et al. 1990; Fujii et al. 1991), accordion-style changes in stack compression have been observed in the neon tetra (Paracheirodon innesi; Clothier and Lythgoe 1987), blue damselfish Chrysiptera cyanea (Oshima and Fujii 1987), ornate tree lizard Urosaurus ornatus (Morrison et al. 1996), and the panther chameleon Furcifer pardalis (Teyssier et al. 2015). Regardless of the type of motion utilized by iridophores, the end result is a shift in the wavelengths of reflected light (Fujii et al. 1991; Goda and Fujii 1998). With respect to reflecting platelet movement and concordant color changes, the terms “aggregation” and “dispersion” are not the most precise descriptors of platelet translocation in iridophores (though they are sufficient when describing pigment movement within melanophores, erythrophores, and leucophores (Fujii 1993; Goda and Fujii 1998)). The different type of intracellular movement that occurs within iridophores prompted Goda and Fujii (1998) to propose the terms “LR response” and “SR response,” where LR and SR are abbreviations for the Longer-wavelength light-Reflecting response and the Shorter-wavelength light-Reflecting response. Loosely, LR corresponds to dispersion or increasing the distance between reflecting platelets (leading to longer-wavelength greens in the blue-green damselfish Chromis viridis; Oshima et al. 1989), and SR to aggregation or contraction of reflecting platelets (leading to shorter-wavelength blues in the blue-green damselfish, C. viridis; Oshima et al. 1989) so as to reduce the space between them (Goda and Fujii 1998).
It is important to note, however, that whether the translocation of reflecting platelets is radial or lateral or the iridophores are dendritic or discoid, all reflecting platelet motility affects the distance between adjacent platelets and, consequently, the wavelength of light reflected by the iridophores (Mäthger et al. 2003). According to Mäthger et al. (2003), the reflection of colored light in the paradise whiptail Pentapodus paradiseus depends on both the thickness of the guanine platelets and the distances between them in the platelet stack. Slight changes in the spacing between the platelets can result in a significant change in color (Teyssier et al. 2015) since the highest reflectivity is observed perpendicular to the surface, when the optical thicknesses (actual thickness * refractive index) of the platelets and spaces are one quarter of the wavelength reflected (Land 1972; Mäthger et al. 2003). Fujii et al. (1991) noted in the dark sleeper (a species of fish Odontobutis obscura) that as reflecting platelets aggregated (SR response), the skin darkened and reflected color shifted from longer to short wavelengths (specifically, yellow to blue). Interestingly, Nagaishi et al. (1990) and Mäthger et al. (2003) also found that the colors reflected by iridophores transitioned hue as the angle of incident light changed from the normal (i.e., the colors were iridescent; Doucet and Meadows 2009). This phenomenon, predicted by the physical properties of reflecting platelets (Huxley 1968; Land 1972) and observed in ex situ skin preparations (Nagaishi et al. 1990; Mäthger et al. 2003), coupled with the observed color changes in living fish suggests that motile reflecting platelets facilitate a secondary method of color change—changing reflecting platelet angle of incidence (Nagaishi et al. 1990). According to this “theory of blinds”, named based on the similarity between changes in platelet angle and the movement of the slats of a Venetian blind, shifts in spectral peaks (i.e., hue and brightness) can also arise due to changes in the orientation of reflecting platelets within the iridophore layer (Nagaishi et al. 1990). In an impressive study, the Venetian blind model has recently been convincingly and empirically validated as a potentially significant force contributing to the rapid color change exhibited by organisms with motile iridophores (Yoshioka et al. 2011).
Relationships with other chromatophores
Since Bagnara et al. (1968) proposed the structure of the dermal chromatophore unit, there has been little deviation from this archtype across diverse taxa of vertebrates capable of physiological color change. The iridophores associated with color change are sequestered beneath a layer of erythrophores and within the upward-extending dendritic processes of melanophores (Bagnara et al. 1968). Although iridophores are usually part of the dermal chromatophore unit, there is no general model which can capture the ratio of iridophores to melanophores (or erythrophores) because the number of iridophores associated with an erythrophore or encased by a melanophore is highly variable (across taxa, within species, and among different regions of the same individual; Bagnara et al. 1968). Although there are many ways in which iridophores differ from melanophores and erythrophores, one peculiar difference is that there is no single description of an iridophore that is representative of them all (Iga et al. 1990; Fujii et al. 1991; Teyssier et al. 2015). That is to say, no one model of an iridophore can capture both the radial and lateral types of reflecting platelet motion, nor account for the observations of iridophores being either non-dendritic, like erythrophores, or dendritic, in which the reflecting platelets disperse into the processes as in melanophores (Iga et al. 1990; Fujii et al. 1991).
In addition to adjusting reflected light on their own, the iridophores have a dynamic and synergistic relationship with the erythrophores and melanophores of the dermal chromatophore unit (Bagnara et al. 1968). Bagnara et al. (1968) investigated the interactions between the iridophores and melanophores of several frog species and found that when melanophores were in the dispersed state, the processes of the melanophores, distended with melanosomes, extend over the top of its associated iridophores thereby preventing light from reaching the reflecting platelets’ reflective surfaces. Conversely, under the influence of melanin aggregating agents (e.g., EPI, norepinephrine, MCH), melanosomes are concentrated (frequently below the iridophores) and contribute less to cell color, particularly because the same agents tend to produce long-wavelength color shifts among iridophores (Kasukawa et al. 1986, 1987; Nagaishi and Oshima 1989; Oshima and Kasai 2002) and enlarge their reflecting area, preventing much light from reaching the melanin (Bagnara et al. 1968). In other words, the translocation of iridophore reflecting platelets is frequently reciprocal to that of melanosomes and the pigments of erythrophores (but not always; Bagnara and Hadley 1969). Pale coloration in vertebrates that undergo physiological color change may, therefore, be dependent on the combined effects of iridophore LR response coupled with melanosome aggregation (Nagaishi and Oshima 1989), whereas skin darkening may primarily the result of melanophore and erythrophore pigment dispersion (Bagnara et al. 1968; Fujii et al. 1991).
Regulation of reflecting platelet motility within iridophores
In exploring the factors that regulate iridophore reflecting platelet motility, many biochemical regulators have been examined based on their well-studied influence on melanophores. For example, one of the first investigations into the hormonal factors influencing platelet movement in amphibians discovered that reflecting platelets aggregated in response to the potent melanophore stimulating agent α-MSH, but were otherwise dispersed to provide the maximum surface area for reflectance (Bagnara et al. 1968). The relationship between α-MSH and reflecting platelet aggregation also appears in several fish species (dark sleeper O. obscura, Iga et al. 1990; Fujii et al. 1991; blue-green damselfish C. viridis, Oshima et al. 1989). However, this relationship is not universal, as the reflecting platelets of ornate tree lizard U. ornatus iridophores do not respond to exogenous administration of MSH (Morrison et al. 1996), nor do those of blue damselfish (C. cyanea, Kasukawa et al. 1987) or common surgeonfish (Paracanthurus hepatus, Goda and Fujii 1998). Additionally, research by Iga et al. (1991) showed that α-MSH-induced aggregation was inhibited in the absence of Ca2+, indicating that platelet motility may depend, as with pterinosomes and carotenoid vesicles, on intracellular Ca2+ concentrations. Maeno and Iga (1992), who found that MSH caused dispersion in melanophores by activating adenylate cyclase and thereby increased intracellular amounts of cAMP, presented evidence that the iridophores of the dark sleeper O. obscura also respond to increasing concentrations of cAMP by aggregating reflecting platelets. Administration of the potent adenylate cyclase activator forskolin or a cAMP analogue (8-bromoadenosine 3′,5′-cyclic monophosphate, or 8-Br-cAMP) resulted in the SR (platelet aggregation) response of reflecting platelets, but whether a decrease in cAMP levels was sufficient for dispersion was not examined (Maeno and Iga 1992). However, Fujii et al. (2000) postulated that the overall result of α-MSH is aggregation in teleostean iridophores and that α-MSH does so by inhibiting adenylate cyclase activity. This claim, when compared with the findings of no effect for MSH on ornate tree lizards, blue damselfish, or common surgeonfish, makes generalizations regarding the effects of α-MSH or changes in cAMP concentrations on translocation of iridophore reflecting platelets impossible across all taxa.
Inconsistent findings regarding the effects of certain hormones or chemicals on reflecting platelet movement within iridophores are not restricted to investigations of α-MSH. There is just as little consistency in the investigations of melatonin and MCH in fish iridophores. Iga et al. (1990) and Oshima et al. (1989) observed the LR (dispersed) response of iridophores after treatment with melatonin in the gobiid dark sleeper O. obscura and blue-green damselfish C. viridis, respectively. Similarly, Oshima et al. (1989) also observed the dispersal of reflecting platelets in response to MCH in C. viridis. However, MCH had no influence on iridophore motility in the blue damselfish C. cyanea (Oshima et al. 1986), and neither melatonin nor MCH had any effect on reflecting platelet motility of the common surgeonfish P. hepatus (Goda and Fujii 1998). This inconsistency, just within teleost fish, illustrates that the effects of MCH, melatonin, and even MSH on iridophore reflecting platelet arrangement are currently indeterminate. Considering the variable effects of MCH, MSH, and melatonin upon the iridophores of different species, it is very likely that the chromatophores of different species or taxa respond differently to various hormones because of inherent variation in hormone receptor types, locations, and abundances.
In stark contrast to the conflicting results concerning α-MSH, MCH, and melatonin, there is consistent agreement regarding the effects of catecholamine neurotransmitters upon fish iridophores. In multiple studies, norepinephrine was found to elicit the dispersive, LR response in the iridophores of teleosts by binding to α-adrenoceptors (Fujii 2000), specifically in the following species: the dark sleeper (O. obscura, Fujii et al. 1991), the freshwater goby (O. obscura, Maeno and Iga 1992), and the common surgeonfish (P. hepatus, Goda and Fujii 1998). Furthermore, Maeno and Iga (1992) determined that the freshwater goby iridophores only possess α-adrenoceptors and that α-adrenoceptor blockers effectively inhibited norepinephrine-induced dispersion (Goda and Fujii 1998).
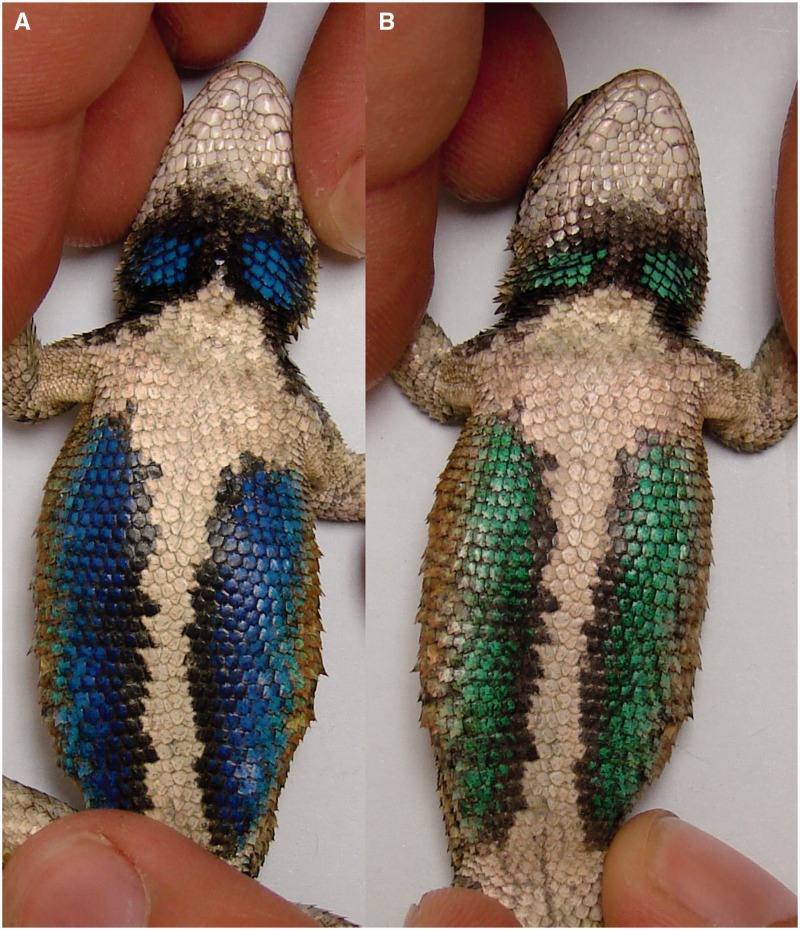
As with melanophores and erythrophores, iridophores of several species also appear to dynamically respond to local environmental conditions. For example, the reflecting platelets of neon tetra P. innesi respond to light by exhibiting the LR response (Lythgoe and Shand 1982). Specifically, Lythgoe and Shand (1982) found that shining a white light on the lateral stripes of tetras caused an increase in the distance between adjacent reflecting platelets and an associated shift toward reflecting more long-wavelength light, even in decapitated fish. Subsequent investigation of neon tetra iridophores uncovered the presence of an opsin-based visual pigment located within the iridophores, suggesting a mechanism for the photoresponsiveness observed in these chromatophores (Lythgoe et al. 1984). In addition to light, the reflecting platelets in ornate tree lizard iridophores have been shown to respond to localized changes in temperature (Morrison et al. 1996). Specifically, heating the ventral, belly of skin of these lizards caused a reduction in the predominant wavelength of reflected light, causing an observed change in color from coppery/green to an intense blue (Morrison et al. 1996). Similar, temperature-dependent changes in structural coloration have been observed in the eastern fence lizard (Sceloporus undulatus, Langkilde and Boronow 2012; Figure 4). Although the specific mechanisms by which increases in temperature alter reflecting platelet spacing in these lizards are currently unknown, it has been postulated that these color changes serve as a signal of thermally dependent performance capability which could be used during intrasexual contests (Langkilde and Boronow 2012).
Figure 4.
Photographs of an individual male eastern fence lizard Sceloporus undulates exhibiting (A) blue coloration at warm temperature (28.8°C) and (B) green coloration at cool temperature (24.6°C).
Note: Photographs courtesy of Tracy Langkilde.
Summary
Evidently, there has been a plethora of research investigating the biochemical regulators of reflecting platelet translocation in fish iridophores, but relatively few investigations of reflecting platelet motility in amphibians or reptiles (with a notable recent exception; Teyssier et al. 2015). Additionally, the specific mechanisms of reflecting platelet translocation are well-understood in just a few species of fish. As in the previous discussions of erythrophores and melanophores, broad generalizations across taxa cannot be made with respect to hormones, neurotransmitters, or other chemicals affecting reflecting platelet translocation. Even the abundant evidence describing fish iridophores is somewhat inconsistent and controversial at larger taxonomic levels, such that generalizations should be limited even within fish. An important exception to this rule is the effect of optical thickness on reflected wavelength (Land 1972; Mäthger et al. 2003) because this relationship addresses physical properties of materials, which are constant despite the organism or cell in which they are found. Although there is potential for further research in many directions, there are three areas that would most clearly benefit from future study. The first would be determining the mechanism for platelet transport, especially given that there are two different kinds of motion, radial and lateral, which have been observed in various iridophores. Next, a more in-depth and consistent understanding of the effects of aggregation and dispersion agonists such as α-MSH, MCH, and melatonin as well as the roles of cAMP and Ca2+ as second messengers would allow clearer discourse when comparing iridophores to other chromatophore types or when discussing the dermal chromatophore unit as a whole. Finally, much as with the erythrophore, there is a relative dearth of comparative information regarding iridophore motility in reptile and amphibian species capable of physiological color change.
Conclusions
Physiological color change is the result of dynamic pigment movement of multiple chromatophores and their interactions with each other within the dermal chromatophore unit. Regulated by hormones, neurotransmitters, and even environmental cues such as light and temperature, chromatophores undergo rapid pigment reorganization to produce a wide array of chromatic changes. Depending on the stimulus, different chromatophore types respond with either dispersion to maximize their relative contribution to the overall color produced, or aggregation to minimize their effect. Here, we have attempted to review the similarities and differences between melanophores, erythrophores, xanthophores, and iridophores, as well as how these different classes of chromatophores can rapidly confer specific colors to the dermis through a coordinated effort.
One important conclusion concerns the reciprocal relationship between iridophores and the other types of chromatophore. Iridophores, located within the basket-like projections of the melanophore, appear to frequently be dispersed to their maximum reflecting surface area when the overlying erythrophores are punctate and the melanophore processes are devoid of melanosomes. This relationship diminishes the colorful contribution of the melanophores and erythrophores and enhances the reflective capacity of the iridophores; this is the light or pale state. In the dark state, iridophores are aggregated and obscured by both the dispersed melanophores and erythrophores; therefore, color absorption is maximized and reflectivity diminished. The likelihood of a general, oppositional response for iridophores, coupled with the sparse literature regarding reptilian and amphibian iridophores, suggests that investigations of the iridophores of all the classes of vertebrates (and their intracellular transduction pathways) would greatly benefit the study of chromatophores and color change. There is also room for future inquiry into the physiological regulation of the erythrophores of reptile and amphibian species, as there is a similar lack of research of their physiological regulation compared with the abundance of information regarding fish erythrophores.
Although our primary focus in this article was to review the current understanding of the cellular and biochemical processes underlying physiological color change, examining color change in an evolutionary context may yield new and exciting insights. Understanding how the ability to rapidly change color evolved, as well as how social and environmental factors might influence the evolution of color change (e.g., Stuart-Fox et al. 2007; Stuart-Fox and Moussalli 2008), could offer key insights into certain mechanisms of chromatophore activity and function. Although we focused on chromatophore responses to particular neural or hormonal signals and excluded discussions of how or why chromatophores are stimulated, determining the reasons for organisms to undergo physiological color change is a crucial next step toward understanding the entire process of chromatophore control and function.
Stuart-Fox and Moussalli (2009) put forth an intriguing review of three of the most prominent functions of physiological color change: camouflage, communication, and thermoregulation. While color change is often used for background matching (Zoond and Eyre 1934; Okelo 1986; Vroonen et al. 2012; Stevens et al. 2014), animals with dynamic color change abilities could potentially employ multiple camouflage strategies in response to different predator types (Stuart-fox et al. 2008; Stuart-Fox and Moussalli 2009). Such facultative, predator-specific crypsis was described by Stuart-Fox and Moussalli (2008) in Smith’s dwarf chameleons Bradypodion taeniabronchum. Using model predators, these authors found that chameleons responded with better background matching for avian predators than for snakes, taking into account the different visual systems of the predators. Whereas the objective of camouflage is to avoid detection or recognition by predators or prey (Stevens and Merilaita 2009), social selection frequently favors conspicuous coloration and rapidly changing color signals for use in intraspecific communication (Stuart-Fox and Moussalli 2009). Animals capable of physiological color change have the benefit of communicating with transient, conspicuous color signals, but may experience reduced predation risk relative to species that display long-term, static coloration or patterns (Stuart-Fox and Moussalli 2009). Therefore, rapid color change can facilitate courtship or be used to signal during aggressive interactions (e.g., Summers and Greenberg 1994; O’Connor et al. 1999; Höglund et al. 2002; Ligon and McGraw 2013; Ligon 2014) without sacrificing the ability to remain inconspicuous after these brief intraspecific encounters (Stuart-Fox and Moussalli 2009).
Color change can also serve in a homeostatic capacity by helping to regulate body temperature (Norris 1967) and, for amphibians, possibly water balance (Stegen et al. 2004; Stuart-Fox and Moussalli 2009). The vertebrates that undergo true physiological color change (i.e., through pigment translocation rather than increased or decreased blood flow) are typically ectothermic, and are thus dependent on external sources of heat for thermoregulation. Ectotherms can thermoregulate via behavior (e.g., shuttling between preferred habitats, Stevenson 1985) or changes in physiology (Whitfield and Livezey 1973), which suggests that another avenue of control over heat absorption may be critical to achieving higher levels of activity at lower temperatures or surviving climates with fluctuating temperatures (Silbiger and Munguia 2008; Stuart-Fox and Moussalli 2009). Darkening the skin increases the amount of light (and heat) absorbed, whereas a lightening of the skin increases reflected light and less heat will be absorbed (Norris 1967; Sherbrooke 1988; Walton and Bennett 1993; Stuart-Fox and Moussalli 2009). For amphibians like frogs, whose skin is freely permeable to water, increases in solar absorbance can also increase the rate of water loss (Stegen et al. 2004). Therefore, the ability to color change in frogs could not only allow them to regulate their body temperature to some degree, but also provide a measure of control over water balance (Stegen et al. 2004).
Although some animals may rely upon all of the aforementioned functions of physiological color change, given the markedly different environments typically inhabited by amphibians, reptiles, and fish, there is undoubtedly competition between the different faculties of color change and each species has likely evolved strategies to address and resolve color change unique to their selective pressures (Stuart-Fox and Moussalli 2009). While the origin of physiological color change in a distant vertebrate ancestor may be difficult to determine, the natural selection for camouflage to avoid predation and sexual selection for signaling capability are possibly the two most important factors in the evolution of color change (Stuart-Fox and Moussalli 2009). Thermoregulation was probably influential for species whose environments demand it; however, compared with camouflage and communication, homeostatic regulation has only recently begun to be considered as a function of physiological color change (Stegen et al. 2004; Stuart-Fox and Moussalli 2009). Therefore, the relative strength of selection exerted by thermoregulatory functions is even more speculative than others. Overall, the extent and specificity of color change with respect to signaling, camouflage, and homeostatic regulation, as well as the interactions and trade-offs between these three functions are currently unknown.
In this review, the physiological control of pigment translocation in the chromatophores and the effects upon the skin color of vertebrates capable of physiological color change were evaluated. In addition to the potential for expanding our knowledge of the regulatory biochemistry of the chromatophores of reptiles, amphibians, and fish, there exists a plethora of new, intriguing research directions related to physiological color change. The questions that remain to be answered involve fully understanding the mechanisms behind pigment organelle locomotion; the evolutionary pathways of color change; determining the importance of and interactions between the various functions of color change; and whether there are previously unknown or unexamined receptors, stimuli, or biochemical pathways that affect chromatophores and physiological color change.
Acknowledgments
The authors thank K.J. McGraw, M.W. Butler, and two anonymous reviewers for providing helpful comments on previous versions of this article. R.A.L. was supported by the ASU Graduate College Completion Fellowship and National Science Foundation Grant no. 1401236 during the creation of this article.
References
- Allen JP, 2008. Biophysical chemistry. West Sussex (UK): Wiley-Blackwell. [Google Scholar]
- Aspengren S, Hedberg D, Sköld HN, Wallin M, 2009. New insights into melanosome transport in vertebrate pigment cells. Int Rev Cell Mol Biol 272:245–302. [DOI] [PubMed] [Google Scholar]
- Bagnara JT, 1966. Cytology and cytophysiology of non-melanophore pigment cells. Int Rev Cytol 38:173–205. [DOI] [PubMed] [Google Scholar]
- Bagnara JT, Hadley ME, 1969. The control of bright colored pigment cells of fishes and amphibians. Integr Comp Biol 9:465–478. [DOI] [PubMed] [Google Scholar]
- Bagnara JT, Hadley ME, 1973. Chromatophores and color change: The comparative physiology of animal pigmentation. Englewood Cliffs (NJ): Prentice-Hall, Inc. [Google Scholar]
- Bagnara JT, Matsumoto J, 2006. Comparative anatomy and physiology of pigment cells in nonmammalian tissues. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS et al., editors. The Pigmentary System: Physiology and Pathophysiology. 2nd edn. Oxford: Blackwell Science, 11–59. [Google Scholar]
- Bagnara JT, Taylor JD, Hadley MACE, 1968. The dermal chromatophore unit. JCell Biol 38:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle MC, Porter KR, 1983. Analysis of the role of microtubules and actin in erythrophore intracellular motility. J Cell Biol 96:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D, Tilney LG, Porter KR, 1966. Microtubules and pigment migration in the melanophores of Fundulus heteroclitus. Protoplasma 61:322–345. [Google Scholar]
- Brown FA, Sandeen MI, 1948. Responses of the chromatophores of the Fiddler crab Uca to light and temperature. Physiol Zool 21:361–371. [DOI] [PubMed] [Google Scholar]
- Byers HR, Porter KR, 1977. Transformations in the structure of the cytoplasmic ground substance in erythrophores during pigment aggregation and dispersion. I. A study using whole-cell preparations in stereo high voltage electron microscopy. J Cell Biol 75:541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Reverter JM, Haitina T, Schiöth HB, Peter RE, 2005. Gene structure of the goldfish agouti-signaling protein: a putative role in the dorsal–ventral pigment pattern of fish. Endocrinology 146:1597–1610. [DOI] [PubMed] [Google Scholar]
- Chatzifotis S, Vaz Juan I, Kyriazi P, Divanach P, Pavlidis M, 2011. Dietary carotenoids and skin melanin content influence the coloration of farmed red porgy (Pagrus pagrus). Aquaculture Nutrition 17:e90–e100. [Google Scholar]
- Clothier J, Lythgoe JN, 1987. Light-induced colour changes by the iridophores of the Neon tetra Paracheirodon innesi. J Cell Sci 88:663–668. [DOI] [PubMed] [Google Scholar]
- Cooper WE, Greenberg N, 1992. Reptilian coloration and behavior. In: Gans C, editor. Biology of the Reptilia; vol. 18. Hormones, Brain, and Behavior. Chicago (IL): University of Chicago Press, 298–422. [Google Scholar]
- de Graan PNE, Van Dorp CJMM, Van De Veerdonk FCG, 1982a. Calcium requirements for α-MSH action on tail-fin melanophores of Xenopus tadpoles. Mol Cell Endocrinol 26:315–326. [DOI] [PubMed] [Google Scholar]
- de Graan PNE, Eberle AN, Van De Veerdonk FCG, 1982b. Calcium sites in MSH stimulation of Xenopus melanophores: Studies with photoreactive α-MSH. Mol Cell Endocrinol 26:327–339. [DOI] [PubMed] [Google Scholar]
- Denton EJ, Land MF, 1971. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc R Soc B 178:43–61. [DOI] [PubMed] [Google Scholar]
- Dorsett D, Yim JJ, Jacobson KB, 1979. Biosynthesis of “drosopterins” by an enzyme system from Drosophila melanogaster. Biochemistry 18:2596–2600. [DOI] [PubMed] [Google Scholar]
- Doucet SM, Meadows MG, 2009. Iridescence: A functional perspective. J R Soc Interface 6(Suppl. 2):S115–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P et al. , 2005. Neuromelanin in human dopamine neurons: Comparison with peripheral melanins and relevance to Parkinson’s disease. Prog Neurobiol 75:109–124. [DOI] [PubMed] [Google Scholar]
- Filadelfi AMC, de Lauro Castrucci AM, 1996. Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates. J Pineal Res 20:175–186. [DOI] [PubMed] [Google Scholar]
- Florey E, 1969. Ultrastructure and function of cephalopod chromatophores. Am Zool 9:429–442. [DOI] [PubMed] [Google Scholar]
- Fox DL, 1976. Animal Biochromes and Structural Colors: Physical, Chemical, Distributional & Physiological Features of Coloured Bodies in the Animal World. 2nd edn. Berkeley (CA): University of California Press. [Google Scholar]
- Fujii R, 1993. Cytophysiology of fish chromatophores. Int Rev Cytol 143:191–255. [Google Scholar]
- Fujii R, 2000. The regulation of motile activity in fish chromatophores. Pigment Cell Res 13:300–319. [DOI] [PubMed] [Google Scholar]
- Fujii R, Hayashi H, Toyohara J, Nishi H, 1991. Analysis of the reflection of light from motile iridophores of the dark sleeper Odontobutis obscura obscura. Zool Sci 8:461–470. [Google Scholar]
- Fujii R, Miyashita Y, 1979. Photoelectric recording of motile responses of fish leucophores. Annot Zool Jpn 52:87–94. [Google Scholar]
- Fujii R, Oshima N, 1986. Control of chromatophore movements in teleost fishes. Zool Sci 3:13–47. [Google Scholar]
- Fukuzawa T, 2004. Unusual leucophore-like cells specifically appear in the lineage of melanophores in the periodic albino mutant of Xenopus laevis. Pigment Cell Res 17:252–261. [DOI] [PubMed] [Google Scholar]
- Goda M, Fujii R, 1998. The blue coloration of the common surgeonfish Paracanthurus hepatus II. Color revelation and color changes. Zool Sci 15:323–333. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Hadley ME, 1970. Evidence for separate receptors for melanophore stimulating hormone and catecholamine regulation of cyclic AMP in the control of melanophore responses. Br J Pharmacol 39:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether GF, Kolluru GR, Nersissian K, 2004. Individual colour patches as multicomponent signals. Biol Rev 79:583–610. [DOI] [PubMed] [Google Scholar]
- Guillot R, Ceinos RM, Cal R, Rotllant J, Cerdá-Reverter JM, 2012. Transient ectopic overexpression of agouti-signalling protein 1 (asip1) induces pigment anomalies in flatfish. PLoS ONE 7:e48526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley ME, Goldman JM, 1969. Physiological color changes in reptiles. Am Zool 9:489–504. [DOI] [PubMed] [Google Scholar]
- Hadley ME, Mieyr JH, Martin BE, de L Castrucci AM, Hruby VJ et al. , 1985. [Nle4, D-Phe7]-α-MSH: A superpotent melanotropin with prolonged action on vertebrate chromatophores. Comp Biochem Physiol A 81:1–6. [DOI] [PubMed] [Google Scholar]
- Hama T, 1963. The relation between the chromatophores and pterin compounds. Ann NY Acad Sci 100:977–986. [PubMed] [Google Scholar]
- Hanlon RT, Messenger JB, 1988. Adaptive coloration in young cuttlefish Sepia officinalis: the morphology and development of body patterns and their relation to behaviour. Philos Trans R Soc B 320:437–487. [Google Scholar]
- Hinton HE, Jarman GM, 1973. Physiological colour change in the elytra of the hercules beetle Dynastes hercules. J Insect Physiol 19:533–549. [Google Scholar]
- Höglund E, Balm PHM, Winberg S, 2002. Behavioural and neuroendocrine effects of environmental background colour and social interaction in Arctic charr Salvelinus alpinus. J Exp Biol 205:2535–2543. [DOI] [PubMed] [Google Scholar]
- Huxley AF, 1968. A theoretical treatment of the reflexion of light by multilayer structures. J Exp Biol 48:227–245. [Google Scholar]
- Ichikawa Y, Ohtani H, Miura I, 1998. The erythrophore in the larval and adult dorsal skin of the brown frog Rana ornativentris: its differentiation, migration, and pigmentary organelle formation. Pigment Cell Melanoma Res 11:345–354. [DOI] [PubMed] [Google Scholar]
- Iga T, Kinutani J, Maeno N, 1990. Motility of cultured iridophores from the freshwater goby Odontobutis obscura. Zool Sci 7:401–407. [Google Scholar]
- Iga T, Takabatake I, 1982. Action of melanophore-stimulating hormone on melanophores of cyrpinid fish Zacco temmincki. Comp Biochem Physiol C 73:51–55. [DOI] [PubMed] [Google Scholar]
- Insausti TC, Casas J, 2008. The functional morphology of color changing in a spider: development of ommochrome pigment granules. J Exp Biol 211:780–789. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K, 2003. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res 16:523–531. [DOI] [PubMed] [Google Scholar]
- Kasukawa H, Oshima N, Fujii R, 1986. Control of chromatophore movements in dermal chromatic units of blue damselfish—II. The motile iridophore. Comp Biochem Physiol C 3:1–7. [DOI] [PubMed] [Google Scholar]
- Kasukawa H, Oshima N, Fujii R, 1987. Mechanism of light reflection in blue damselfish motile iridophore. Zool Sci 4:243–257. [Google Scholar]
- Kotz KJ, McNiven MA, 1994. Intracellular calcium and cAMP regulate directional pigment movements in teleost erythrophores. J Cell Biol 124:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF, 1972. The physics and biology of animal reflectors. Prog Biophys Mol Biol 24:75–106. [DOI] [PubMed] [Google Scholar]
- Langkilde T, Boronow KE, 2012. Hot boys are blue: temperature-dependent color change in male eastern fence lizards. J Herpetol 46:461–465. [Google Scholar]
- Larson ET, Summers CH, 2001. Serotonin reverses dominant social status. Behav Brain Res 121:95–102. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C, 1999. The Neural Crest. Cambridge: Cambridge University Press. [Google Scholar]
- Lerner AB, Fitzpatrick TB, 1950. Biochemistry of melanin formation. Physiol Rev 30:91–126. [DOI] [PubMed] [Google Scholar]
- Ligon RA, 2014. Defeated chameleons darken dynamically during dyadic disputes to decrease danger from dominants. Behav Ecol Sociobiol 68:1007–1017. [Google Scholar]
- Ligon RA, McGraw KJ, 2013. Chameleons communicate with complex colour changes during contests: different body regions convey different information. Biol Lett 9:20130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DW, Burn SF, Jackson IJ, 2006. Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res 19:206–213. [DOI] [PubMed] [Google Scholar]
- Lythgoe JN, Shand J, 1982. Changes in spectral reflexions from the iridophores of the neon tetra. J Physiol 325:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythgoe JN, Shand J, Foster RG, 1984. Visual pigment in fish iridocytes. Nature 308:83–84. [Google Scholar]
- Maeno N, Iga T, 1992. Adrenergic mechanisms associated with the movement of platelets in iridophores from the freshwater goby Odontobutis obscura. Comp Biochem Physiol C 102:233–237. [DOI] [PubMed] [Google Scholar]
- Marsden CD, 1961. Pigmentation in the nucleus substantia nigra of mammals. J Anat 95:256–261. [PMC free article] [PubMed] [Google Scholar]
- Mäthger LM, Land MF, Siebeck UE, Marshall NJ, 2003. Rapid colour changes in multilayer reflecting stripes in the paradise whiptail Pentapodus paradiseus. J Exp Biol 33:3607–3613. [DOI] [PubMed] [Google Scholar]
- McGraw K, 2006. Mechanics of carotenoid-based coloration. In: Hill G, McGraw K, editors. Bird Color; vol. I. Mechanisms and Measurements. Cambridge (MA): Harvard University Press, 177–242. [Google Scholar]
- Menter DG, Obika M, Tchen TT, Taylor JD, 1979. Leucophores and iridophores of Fundulus heteroclitus: biophysical and ultrastructural properties. J Morphol 160:103–119. [DOI] [PubMed] [Google Scholar]
- Messenger JB, 2001. Cephalopod chromatophores: neurobiology and natural history. Biol Rev 76:473–528. [DOI] [PubMed] [Google Scholar]
- Møller AP, Biard C, Blount JD, Houston DC, Ninni P et al. , 2000. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult Biol Rev 11:137–159. [Google Scholar]
- Morrison RL, Rand MS, Frost-mason SK, 1995. Cellular basis of color differences in three morphs of the lizard Sceloporus undulatus erythrocheilus. Copeia 1995:397–408. [Google Scholar]
- Morrison RL, Sherbrooke WC, Frost-Mason SK, 1996. Temperature-sensitive, physiologically active iridophores in the lizard Urosaurus ornatus: an ultrastructural analysis of color change. Copeia 1996:804–812. [Google Scholar]
- Nagaishi H, Oshima N, 1989. Neural control of motile activity of light-sensitive iridophores in the neon tetra. Pigment Cell Melanoma Res 2:485–492. [DOI] [PubMed] [Google Scholar]
- Nagaishi H, Oshima N, Fujii R, 1990. Light-reflecting properties of the iridophores of the neon tetra Paracheirodon innesi. Comp Biochem Physiol A 95:337–341. [Google Scholar]
- Nelson DL, Cox MM, 2008. Lehninger Principles of Biochemistry. 5th edn. New York: W.H. Freeman and Co. [Google Scholar]
- Nery LEM, Castrucci AMD, 1997. Pigment cell signalling for physiological color change. Comp Biochem Physiol A 29:1135–1144. [DOI] [PubMed] [Google Scholar]
- Nicolaus RA, Piattelli M, 1962. Structure of melanins and melanogenesis. J Polym Sci 58:1133–1139. [Google Scholar]
- Nielsen HI, 1978. Ultrastructural changes in the dermal chromatophore unit of Hyla arborea during color change. Cell Tissue Res 194:405–418. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Wallin M, 1997. Evidence for several roles of dynein in pigment transport in melanophores. Cell Motil Cytoskeleton 38:397–409. [DOI] [PubMed] [Google Scholar]
- Nilsson Sköld H, Aspengren S, Wallin M, 2013. Rapid color change in fish and amphibians: function, regulation, and emerging applications. Pigment Cell Melanoma Res 26:29–38. [DOI] [PubMed] [Google Scholar]
- Nishi H, Fujii R, 1992. Novel receptors for melatonin that mediate pigment dispersion are present in some melanophores of the pencil fish Nannostomus. Comp Biochem Physiol C 103:263–268. [Google Scholar]
- Norris KS, 1967. Color adaptation in desert reptiles and its thermal relationships. In: Milstead WW, editor. Lizard Ecology: a Symposium. Columbia: University of Missouri Press, 162–229. [Google Scholar]
- Novales RR, Novales BJ, 1965. The effects of osmotic pressure and calcium deficiency on the response of tissue-cultured melanophores to melanocyte-stimulating hormone. Gen Comp Endocrinol 5:568–576. [DOI] [PubMed] [Google Scholar]
- O’Connor KIK, Metcalfe NBNNBN, Taylor ACAACA, 1999. Does darkening signal submission in territorial contests between juvenile Atlantic salmon Salmo salar? Anim Behav 58:1269–1276. [DOI] [PubMed] [Google Scholar]
- Obika M, 1963. Association of pteridines with amphibian larval pigmentation and their biosynthesis in developing chromatophores. Dev Biol 6:99–112. [DOI] [PubMed] [Google Scholar]
- Obika M, 1988. Ultrastructure and physiological response of leucophores of the medaka Oryzias latipes. Zool Sci 5:311–321. [Google Scholar]
- Obika M, Bagnara JT, Science S, Series N, Jan N, 1964. Pteridines as pigments in amphibians. Science 143:485–487. [DOI] [PubMed] [Google Scholar]
- Okelo O, 1986. Neuroendocrine control of physiological color change in Chameleo gracilis. Gen Comp Endocrinol 64:305–311. [DOI] [PubMed] [Google Scholar]
- Oshima N, Fujii R, 1987. Motile mechanism of blue damselfish Chrysiptera cyanea iridophores. Cell Motil Cytoskeleton 8:85–90. [Google Scholar]
- Oshima N, Kasai A, 2002. Iridophores involved in generation of skin color in the zebrafish Brachydanio rerio. Forma 17:91–101. [Google Scholar]
- Oshima N, Kasukawa H, Fujii R, 1989. Control of chromatophore movements in the blue-green damselfish Chromis viridis. Comp Biochem Physiol C 93:239–245. [Google Scholar]
- Oshima N, Kasukawa H, Fujii R, Wilkes BC, Hruby VJ et al. , 1986. Action of melanin-concentrating hormone (MCH) on teleost chromatophores. Gen Comp Endocrinol 64:381–388. [DOI] [PubMed] [Google Scholar]
- Oshima N, Nakamaru N, Araki S, Sugimoto M, 2001. Comparative analyses of the pigment-aggregating and -dispersing actions of MCH on fish chromatophores. Comp Biochem Physiol C 129:75–84. [DOI] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M, 1996. Colour vision as an adaptation to frugivory in primates. Proc R Soc B Biol Sci 263:593–599. [DOI] [PubMed] [Google Scholar]
- Prum RO, 2006. Anatomy, physics, and evolution of structural colors. In: Hill GE, McGraw KJ, editors. Bird Color; vol. I. Mechanisms and Measurements. Cambridge (MA): Harvard University Press, 295–353. [Google Scholar]
- Rhodes L, Argersinger ME, Gantert LT, Friscino BH, Hom G et al. , 1997. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, an aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J Reprod Fertil 111:51–57. [DOI] [PubMed] [Google Scholar]
- Rodionov V, Yi J, Kashina A, Oladipo A, Gross SP, 2003. Switching between microtubule- and actin-based transport systems in melanophores is controlled by cAMP levels. Curr Biol 13:1837–1847. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Gelfand VI, 1991. Kinesin is responsible for centrifugal movement of pigment granules in melanophores. Proc Natl Acad Sci USA 88:4956–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollag MD, 1988. Response of amphibian melanophores to melatonin. In: Reiter RJ, editor. Pineal Research Reviews. vol. 6. New York: Alan R. Liss, 67–93. [Google Scholar]
- Roulin A, Mafli A, Wakamatsu K, 2013. Reptiles produce pheomelanin: evidence in the eastern Hermann’s tortoise Eurotestudo boettgeri. J Herpetol 47:258–261. [Google Scholar]
- Rozdzial MM, Haimo LT, 1986. Reactivated melanophore motility: differential regulation and nucleotide requirements of bidirectional pigment granule transport. J Cell Biol 103:2755–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak PJ, Adams SR, Harootunian AT, Schliwa M, Tsien RY, 1992. Intracellular cyclic AMP not calcium, determines the direction of vesicle movement in melanophores: direct measurement by fluorescence ratio imaging. J Cell Biol 117:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake N, 1980. Effect of methionine–enkephalin on xanthophore aggregation. Peptides 1:73–75. [DOI] [PubMed] [Google Scholar]
- Sato M, Ishikura R, Oshima N, 2004. Direct effects of visible and UVA light on pigment migration in erythrophores of Nile tilapia. Pigment Cell Res 17:519–524. [DOI] [PubMed] [Google Scholar]
- Seiji M, Fitzpatrick TB, Birbeck MS, 1961. The melanosome: a distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis. J Invest Dermatol 36:243–252. [DOI] [PubMed] [Google Scholar]
- Seiji M, Shimaoi K, Birbeckz MSC, Fitzpatrick TB, 1963. Subcellular localization of melanin biosynthesis. Ann NY Acad Sci 100:497–533. [PubMed] [Google Scholar]
- Serway RA, Vuille C, Faughn JS, 2009. College Physics. 8th edn. Belmont (TN): Brooks/Cole Cengage Learning. [Google Scholar]
- Sherbrooke WC, 1988. Integumental Biology of Horned Lizards. Tucson (AZ): University of Arizona. [Google Scholar]
- Silbiger N, Munguia P, 2008. Carapace color change in Uca pugilator as a response to temperature. J Exp Mar Biol Ecol 355:41–46. [Google Scholar]
- Stackhouse HL, 1966. Some aspects of pteridine biosynthesis in amphibians. Comp Biochem Physiol 17:219–235. [DOI] [PubMed] [Google Scholar]
- Stegen JC, Gienger CM, Sun L, 2004. The control of color change in the Pacific tree frog, Hyla regilla. Can J Zool 82:889–896. [Google Scholar]
- Stevens M, Lown AE, Denton AM, 2014. Rockpool gobies change colour for camouflage. PLoS ONE 9:e110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M, Merilaita S, 2009. Animal camouflage: current issues and new perspectives. Philos Trans R Soc Lond B Biol Sci 364:423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RD, 1985. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am Nat 126:362–386. [Google Scholar]
- Stuart-Fox D, Moussalli A, 2008. Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol 6:e25 doi:10.1371/journal.pbio.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Fox D, Moussalli A, 2009. Camouflage, communication and thermoregulation: lessons from colour changing organisms. Philos Trans R Soc B 364:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Fox D, Moussalli A, Whiting MJ, 2007. Natural selection on social signals: signal efficacy and the evolution of chameleon display coloration. Am Nat 170:916–930. [DOI] [PubMed] [Google Scholar]
- Stuart-fox D, Moussalli A, Whiting MJ, 2008. Predator-specific camouflage in chameleons. Biol Lett 4:326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga T, Munesada K, 1988. The pigments in the dorsal skin of frogs. J Nat Prod 51:713–718. [DOI] [PubMed] [Google Scholar]
- Sugden D, 1991. Aggregation of pigment granules in single cultured Xenopus laevis melanophores by melatonin analogues. Br J Pharmacol 104:922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Greenberg N, 1994. Somatic correlates of adrenergic activity during aggression in the lizard Anolis carolinensis. Horm Behav 28:29–40. [DOI] [PubMed] [Google Scholar]
- Svensson PA, Wong BBM, 2011. Carotenoid-based signals in behavioural ecology: a review. Behaviour 148:131–189. [Google Scholar]
- Takeuchi IK, 1976. Electron microscopy of two types of reflecting chromatophores (iridophores and leucophores) in the guppy Lebistes reticulatus Peters. Cell Tissue Res 173:17–27. [DOI] [PubMed] [Google Scholar]
- Taylor JD, 1969. The effects of intermedin on the ultrastructure of amphibian iridophores. Gen Comp Endocrinol 12:405–416. [DOI] [PubMed] [Google Scholar]
- Teyssier J, Saenko SV, van der Marel D, Milinkovitch MC, 2015. Photonic crystals cause active colour change in chameleons. Nat Commun 6:6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma MC, Gelfand VI, 1999. Molecular mechanisms of pigment transport in melanophores. Pigment Cell Res 12:283–294. [DOI] [PubMed] [Google Scholar]
- Umbers KDL, Fabricant SA, Gawryszewski FM, Seago AE, Herberstein ME, 2014. Reversible colour change in Arthropoda. Biol Rev Camb Philos Soc 89:820–848. [DOI] [PubMed] [Google Scholar]
- Van De Veerdonk FC, Brouwer E, 1973. Role of calcium and prostaglandin (PGE) in the MSH-induced activation of adenylate cyclase in Xenopus laevis. Biochem Biophys Res Commun 52:130–136. [DOI] [PubMed] [Google Scholar]
- Vaughan GL, Greenberg N, 1987. Propanolol, a beta-adrenergic antagonist, retards response to MSH in skin of Anolis carolinensis. Physiol Behav 40:555–558. [DOI] [PubMed] [Google Scholar]
- Vroonen J, Vervust B, Fulgione D, Maselli V, Van Damme R, 2012. Physiological colour change in the Moorish gecko Tarentola mauritanica (Squamata: Gekkonidae): effects of background, light, and temperature. Biol J Linn Soc 107:182–191. [Google Scholar]
- Walton BM, Bennett AF, 1993. Temperature-dependent color change in Kenyan chameleons. Physiol Zool 66:270–287. [Google Scholar]
- Weiss SL, Foerster K, Hudon J, 2012. Pteridine, not carotenoid, pigments underlie the female-specific orange ornament of striped plateau lizards Sceloporus virgatus. Comp Biochem Physiol B 161:117–123. [DOI] [PubMed] [Google Scholar]
- Whitfield CL, Livezey RL, 1973. Thermoregulatory patterns in lizards. Physiol Zool 46:285–296. [Google Scholar]
- Wilkes BC, Hruby VJ, Sherbrooke WC, Castrucci AM de L, Hadley ME, 1984. Synthesis and biological actions of melanin concentrating hormone. Biochem Biophys Res Commun 122:613–619. [DOI] [PubMed] [Google Scholar]
- Wright MR, Lerner AB, 1960. On the movement of pigment granules in frog melanocytes. Endocrinology 66:599–609. [DOI] [PubMed] [Google Scholar]
- Yoshioka S, Matsuhana B, Tanaka S, Inouye Y, Oshima N et al. , 2011. Mechanism of variable structural colour in the neon tetra: quantitative evaluation of the Venetian blind model. J R Soc Interface 8:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Song Y, Thompson DA, Madonna MA, Millhauser GL. et al. , 2010. Pineal-specific agouti protein regulates teleost background adaptation. Proc Natl Acad Sci USA 107:20164–20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler I, 2003. The pteridine pathway in zebrafish: regulation and specification during the determination of neural crest cell-fate. Pigment Cell Res 16:172–182. [DOI] [PubMed] [Google Scholar]
- Zoond A, Eyre J, 1934. Studies in reptilian colour response. I. The bionomics and physiology of the pigmentary activity of the chameleon. Phil Trans R Soc B 223:27–55. [Google Scholar]