Table 1. One-pot routes to rac-2, (S)-trolline 2 and analogues via step a and steps a + b.
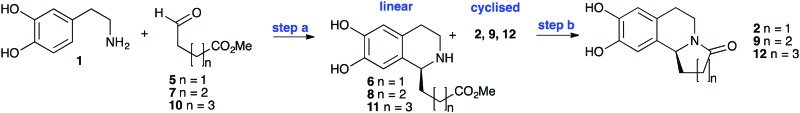
| ||||||
| Aldehyde | Solvent | Step a
a
or
f
|
Steps a + b
b
|
|||
| Linear product yield c (para : ortho) | Cyclised product yield c (para : ortho) | Product yield c (para : ortho) | Isolated product yield (para : ortho) | Product ee h | ||
| KPi step a a | ||||||
| 5 | 50% CH3CN/KPi | 6 32% (15 : 1) | 2 68% (22 : 1) | 2 97% (18 : 1) | 2 81% (34 : 1) d | na |
| 7 | 50% CH3CN/KPi | 8 75% (8 : 1) | 9 14% (13 : 1) | 9 89% (9 : 1) | 9 69% (50 : 1) e | na |
| 10 | 50% CH3CN/KPi | 11 72% (7 : 1) | Only 11 | 11 72% (7 : 1) | 11 26% (7 : 1) e | na |
| Enzymatic step a f | ||||||
| 5 g | 10% CH3CN/HEPES | 6 35% | 2 3% | na | na | nd |
| 5 g | 10% DMSO/HEPES | 6 61% | 2 7% | na | na | nd |
| 5 | 10% DMSO/HEPES | 6 71% | 2 15% | na | na | nd |
| 5 | 1% DMSO/HEPES | 6 67% | 2 19% | 2 75% | 2 74% d | 95% |
| 7 | 1% DMSO/HEPES | 8 82% | 9 15% | 9 96% | 9 87% d | 96% |
| 10 | 1% DMSO/HEPES | 11 92% | only 11 | 11 92% | 11 63% e | >99% |
a 1 and aldehyde (1 : 1.5) in KPi buffer (0.3 M)/CH3CN (1 : 1), 18 h, under Ar, 60 °C, pH 6, and ascorbic acid (1 equiv.).
bNa2CO3 (1 M), pH 7.5, and 4 h.
cHPLC yields: calculated by analytical HPLC.
dIsolated in high purity by a basic and then acidic extraction procedure using EtOAc and then MeOCO2Me.
eIsolated by preparative HPLC.
f 1 and aldehyde (ratio 1 : 1.5) in co-solvent/HEPES buffer (pH 7.5 and 0.1 M), 37 °C, WT-TfNCS (0.1 mg mL–1), sodium ascorbate (1 equiv.), and 6 h.
gAs for f but 1 : 5 in a ratio of 1.5 : 1, and 3 h reaction.
hThe ees determined by chiral HPLC: for trolline the absolute stereochemistry was confirmed by the optical rotation. na, not applicable. nd, not determined.
