For the current issue of the Journal, we asked Drs. Alexandra Faber, Lagman-Bartolome and Rajapakse to comment on and put into context the recent Cochrane Review on drugs for the acute treatment of migraine in children and adolescents.
BACKGROUND
Numerous medications are available for the acute treatment of migraine in adults, and some have now been approved for use in children and adolescents in the ambulatory setting. A systematic review of acute treatment of migraine medication trials in children and adolescents will help clinicians make evidence-informed management choices.
OBJECTIVES
To assess the effects of pharmacological interventions by any route of administration versus placebo for migraine in children and adolescents 17 years of age or less. For the purposes of this review, children were defined as under 12 years of age and adolescents 12–17 years of age.
SEARCH METHODS
We searched seven bibliographic databases and four clinical trial registers as well as gray literature for studies through February 2016.
SELECTION CRITERIA
We included prospective randomized controlled clinical trials of children and adolescents with migraine, comparing acute symptom relieving migraine medications with placebo in the ambulatory setting.
DATA COLLECTION AND ANALYSIS
Two reviewers screened titles and abstracts and reviewed the full text of potentially eligible studies. Two independent reviewers extracted data for studies meeting inclusion criteria. We calculated the risk ratios (RRs) and number needed to treat for an additional beneficial outcome (NNTB) for dichotomous data. We calculated the risk difference (RD) and number needed to treat for an additional harmful outcome (NNTH) for proportions of adverse events. The percentage of pain-free patients at 2 hours was the primary efficacy outcome measure. We used adverse events to evaluate safety and tolerability. Secondary outcome measures included headache relief, use of rescue medication, headache recurrence, presence of nausea and presence of vomiting. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created ‘Summary of findings’ tables.
MAIN RESULTS
We identified a total of 27 randomized controlled trials (RCTs) of migraine symptom-relieving medications, in which 9158 children and adolescents were enrolled and 7630 (range of mean age between 8.2 and 14.7 years) received medication. Twenty-four studies focused on drugs in the triptan class, including almotriptan, eletriptan, naratriptan, rizatriptan, sumatriptan, sumatriptan + naproxen sodium and zolmitriptan. Other medications studied included paracetamol (acetaminophen), ibuprofen and dihydroergotamine. More than half of the studies evaluated sumatriptan. All but one study reported adverse event data. Most studies presented a low or unclear risk of bias, and the overall quality of evidence, according to GRADE criteria, was low to moderate, downgraded mostly due to imprecision and inconsistency. Ibuprofen was more effective than placebo for producing pain freedom at 2 hours in two small studies that included 162 children (RR 1.87, 95% confidence interval [CI] 1.15 to 3.04) with low quality evidence (due to imprecision). Paracetamol was not superior to placebo in one small study of 80 children. Triptans as a class of medication were superior to placebo in producing pain freedom in three studies involving 273 children (RR 1.67, 95% CI 1.06 to 2.62, NNTB 13) (moderate quality evidence) and 21 studies involving 7026 adolescents (RR 1.32, 95% CI 1.19 to 1.47, NNTB 6) (moderate quality evidence). There was no significant difference in the effect sizes between studies involving children versus adolescents. Triptans were associated with an increased risk of minor (nonserious) adverse events in adolescents (RD 0.13, 95% CI 0.08 to 0.18, NNTH 8), but studies did not report any serious adverse events. The risk of minor adverse events was not significant in children (RD 0.06, 95% CI –0.04 to 0.17, NNTH 17). Sumatriptan plus naproxen sodium was superior to placebo in one study involving 490 adolescents (RR 3.25, 95% CI 1.78 to 5.94, NNTB 6) (moderate quality evidence). Oral dihydroergotamine was not superior to placebo in one small study involving 13 children.
AUTHORS’ CONCLUSIONS
Low quality evidence from two small trials shows that ibuprofen appears to improve pain freedom for the acute treatment of children with migraine. We have only limited information on adverse events associated with ibuprofen in the trials included in this review. Triptans as a class are also effective at providing pain freedom in children and adolescents but are associated with higher rates of minor adverse events. Sumatriptan plus naproxen sodium is also effective in treating adolescents with migraine.
The full text of the Cochrane Review is available in The Cochrane Library: Richer L, Billinghurst L, Linsdell MA, Russell K, Vandermeer B, Crumley ET, Durec T, Klassen TP, Hartling L. Drugs for the acute treatment of migraine in children and adolescents. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No.: CD005220. DOI: 10.1002/14651858.CD005220.pub2.
EXPERT COMMENTARY
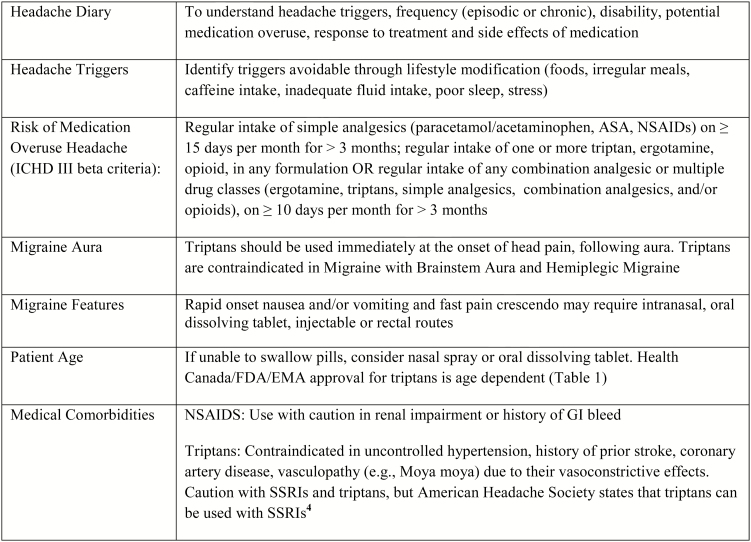
Currently, no Canadian guidelines exist for the management of paediatric migraine and American guidelines are under review (1). Further paediatric and adolescent acute migraine treatment trials and clinical practice guidelines are needed to provide evidence based care. Nearly 1 in 10 children experience significant headaches (migraine or tension type) between the ages of 5–15 years (2). Diagnostic criteria for migraine without aura and paediatric considerations can be found in the International Classification of Headache Disorders version III Beta (3). Effective management requires education about migraines (Appendix 1) and development of an individualized acute treatment plan in conjunction with a health care provider (see Figure 1). Many safe treatment options for children exist, and combinations of medications can be safely used.
Figure 1.
Elements of an individualized acute migraine treatment plan. EMA European Medicines Agency; FDA Food and Drug Administration.
Pharmacological abortive therapies should be used early in the pain phase when efficacy is highest. The recommended therapeutic dose, acceptable for weight, should be used to avoid treatment ‘failure’. There is no evidence to suggest that only shorter acting agents should be used in younger populations. There is no evidence to support using nonmigraine-specific medications (NSAIDs, acetaminophen) as first-line therapy in children over migraine specific (i.e., triptan) therapy, however the tolerability, affordability and availability of nonmigraine-specific medications, allows for them to be tried as first-line treatments (1). The doses should be maximized and include ibuprofen (10 mg/kg/dose), acetaminophen (15 mg/kg/dose), or naproxen sodium (7–10 mg/kg/dose). Given the lack of evidence for what is best for first-line treatment, migraine-specific medications (i.e., triptans) can also be considered for use as first-line treatments, or after failure of nonmigraine-specific medications (1). Health Canada approval exists for seven triptans in adults, and one triptan (almotriptan) in children (≥12 years old). In children, there are four triptans with Food and Drug Administration approval, and one additional triptan with European Medicines Agency approval (Table 1). Intranasal Sumatriptan has been shown to be effective, in acute migraine treatment of adolescents in a randomized controlled trial (8). As well, in the current Cochrane Review subgroup analysis, intranasal triptans (sumatriptan and zolmitriptan) produced higher effect sizes than all oral triptans in the meta analysis, but the evidence was inconsistent (9). The combination of sumatriptan 85 mg and naproxen sodium 500 mg has shown efficacy in the adolescent population, and perhaps other combination treatments can work for patients on an individualized basis, however further data is required to support this model of synergistic treatment in adolescents (10). Pharmacologically, simple analgesics (NSAIDs, acetaminophen) and triptans do not have drug interactions and are medically safe to combine at the onset of a severe debilitating headache. While data to support the benefit of oral antiemetic add on therapy is currently lacking, based on IV antiemetic studies there may be a benefit to trialing oral antiemetics (ondansetron, metoclopramide) in patients requiring relief from nausea and vomiting in addition to migraine (11–14).
Table 1.
Triptan agents with Paediatric Health Canada and/or FDA Approval
| Triptan | Route | Approval agency (year approved) | Age | Dose |
|---|---|---|---|---|
| Rizatriptan | Oral Dissolving Tablet | FDA (2011) | ≥6 years old | 5 mg (<40 kg), 10 mg (≥40 kg) |
| Zolmitriptan | Nasal Spray (Oral dissolving tablet not approved) | FDA (2015) | ≥12 years old | 2.5 mg, 5 mg |
| Almotriptan | Oral Tablet | Health Canada (2011), FDA (2009) | ≥12 years old | 12.5 mg |
| Sumatriptan | Nasal Spray | EMA (2015) | ≥12 years old (Age 6 years old, AAN 2004 statement) | 10 mg (20 mg, not approved) |
| Sumatriptan + Naproxen Sodium (Not available in Canada) | Oral tablet | FDA (2015) | ≥ 12 years old | Sumatriptan 85 mg + Naproxen Sodium 500 mg |
Data taken from refs. (5,6,7)
EMA European Medicines Agency; FDA Food and Drug Administration; AAN American Academy of Neurology
In children, there is no evidence to suggest that ibuprofen or paracetamol/acetaminophen are better than triptans. The objective of the Cochrane review was to compare acute pharmacological interventions to placebo in the paediatric population. The review did not address the relative efficacy of simple analgesics as compared to triptans. There are two three way cross over studies showing that ibuprofen was not superior to zolmitriptan or paracetamol but these were small studies and little data exists on this topic (15,16). Most paediatric RCTs in migraine compare ibuprofen to placebo. Further RCTs comparing ibuprofen to triptans in the paediatric population specifically are required. The Cochrane review highlights that triptans are safe and well tolerated in the paediatric and adolescent population with an observed increase in minor adverse events (‘taste disturbances, visual symptoms, dizziness, fatigue, low energy, nausea or vomiting’) but no significant increase in serious adverse events in adolescents (9). Side effects from one triptan do not preclude trying a different triptan. The safety data compiled in this Cochrane review provides reassurance about the tolerability of triptans in the paediatric population and should encourage primary care providers and paediatricians to consider their use in patients without contraindications.
Overuse of triptans (defined above) can cause medication overuse headache and is a risk factor for chronification of migraine (17). Limiting the number of days per month where acute treatment is with a pharmacologic intervention and teaching children how to manage pain through nonpharmacological approaches is critical in headache management. Proven strategies include cognitive behaviour therapy and biofeedback (18–22).
In Canadian paediatric emergency departments, 2.2% of presenting patients had used a triptan and 4.7% had used an opioid (23). It may be that the triptan was taken too late after the start of a headache, but this data may also suggest that a low percentage of patients are trying migraine specific abortive treatments prior to presenting to the Emergency department. It is also concerning that opioids are being used in children for migraine. Opioids can promote migraine chronification (in adults) and have a strong potential for abuse. Further, the American Academy of Neurology’s top five choosing wisely recommendations include avoiding opioids for treatment of migraine, ‘except as a last resort’ (24). There are recent Food and Drug Administration warnings for tramadol use in children with pain (5). This data should support primary care providers and paediatricians in prescribing nonopioid treatments to children for acute migraine treatment (25).
In managing paediatric migraine, we must consider complementary nonpharmacological daily headache prevention strategies in conjunction with pharmacological headache management, include avoiding medication overuse and dehydration, a regular sleep schedule, avoiding or limiting caffeine, eating regular meals, eating high protein in the morning, regular exercise, stress management and a trial of migraine supplements (26–28). A review of nutraceuticals, for migraine prevention in children, made a weak recommendation upon low quality evidence for the use of magnesium, coenzyme Q10 and butterbur (28,29). Given that the quality of evidence for the use of nutraceuticals in paediatric migraine is poor with some risk of harm (some butterbur supplements have resulted in hepatotoxicity due to pyrrolizidine alkaloids), and the cost burden can be high, nutraceuticals may not be ideal for some patients (28,29). Pharmacological daily headache prevention can be useful in management of paediatric headache, however it is also important to note that a recent 2017 randomized double-blind placebo controlled trial of amitriptyline, topiramate and placebo showed no significant between group differences and higher rates of several adverse events in the pharmacological intervention groups (30). This study highlights the need for improved therapeutic options with favourable side effect profiles in paediatric headache. The significant placebo effect frequently seen in paediatric migraine clinical trials suggests that paediatric migraine is not simply a variant of adult migraine but may have a distinct neurobiological origin requiring more targeted therapies. Paediatric migraine may potentially be more responsive to behavioural interventions which can be very effective and are well tolerated. Ultimately this negative study reinforces the need for development of pharmacological ‘best practice’ guidelines for paediatric migraine prevention as well as exploration of targeted investigational therapies that are gaining momentum in migraine research such as neurostimulation and monoclonal antibodies (31).
We are encouraged by the Cochrane review on drugs for the acute treatment of migraine in children and adolescents, as it provides evidence for the efficacy and safety of prescribing ibuprofen and triptans for the acute relief of paediatric migraine. The review also highlights the need for ongoing research in this population. The authors have expertly brought existing paediatric evidence to the forefront to aid in knowledge translation and improve care in this disabling medical condition.
Conflict of Interest: AML-B reports grants received from Allergan and from Tribute outside the submitted work.
Appendix 1. Web-based resources for headache education and management
-
Adolescent websites and apps for headache and stress management
http://headachereliefguide.com/index.php (accessed October 9, 2017)
http://mindfulnessforteens.com/ (accessed October 9, 2017)
Mindshift Website and App: https://www.anxietybc.com/resources/mindshift-app (accessed October 9, 2017)
Stop, Breathe and Think App: https://itunes.apple.com/us/app/stop-breathe-think/id778848692?mt=8 (accessed October 9, 2017)
Smiling Mind Website and App: https://smilingmind.com.au/ (accessed October 9, 2017)
-
Resources for clinicians and patients including handouts
https://www.ichd-3.org (accessed October 9, 2017)
http://migrainecanada.org/ (accessed October 9, 2017)
https://americanheadachesociety.org/ (accessed October 9, 2017)
https://migraine.com/ (accessed October 9, 2017)
-
Headache apps:
iHeadache app: https://itunes.apple.com/us/app/iheadache-free-headache-migraine-diary-app/id374213833?mt=8 (accessed October 9, 2017)
Migraine Buddy App: https://itunes.apple.com/us/app/migraine-buddy/id975074413?mt=8 (accessed October 9, 2017)
-
Headache Diaries:
References
- 1. Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S; American Academy of Neurology Quality Standards Subcommittee; Practice Committee of the Child Neurology Society Practice parameter: Pharmacological treatment of migraine headache in children and adolescents: Report of the American academy of neurology quality standards subcommittee and the practice committee of the child neurology society. Neurology 2004;63(12):2215–24. [DOI] [PubMed] [Google Scholar]
- 2. Abu-Arefeh I, Russell G. Prevalence of headache and migraine in schoolchildren. BMJ 1994;309(6957):765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Headache Classification Committee of the International Headache Society (IHS). The international Classification of Headache Disorders, 3rd edn (beta version). Cephalalgia 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- 4. Evans RW, Tepper SJ, Shapiro RE, Sun-Edelstein C, Tietjen GE. The fda alert on serotonin syndrome with use of triptans combined with selective serotonin reuptake inhibitors or selective serotonin-norepinephrine reuptake inhibitors: American headache society position paper. Headache 2010;50(6):1089–99. [DOI] [PubMed] [Google Scholar]
- 5. Langer-Gould AM, Anderson WE, Armstrong MJ et al. The American academy of neurology’s top five choosing wisely recommendations. Neurology 2013;81(11):1004–11. [DOI] [PubMed] [Google Scholar]
- 6. Health Canada Drugs and Health Products http://www.hc-sc.gc.ca/dhp-mps/prodpharma/index-eng.php. (Accessed October 9, 2017).
- 7. European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/home/Home_Page.jsp&mid=. (Accessed October 9, 2017).
- 8. Winner P, Rothner AD, Saper J et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics 2000;106(5):989–97. [DOI] [PubMed] [Google Scholar]
- 9. Richer L, Billinghurst L, Linsdell MA et al. Drugs for the acute treatment of migraine in children and adolescents. Cochrane Database Syst Rev 2016;4:CD005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Derosier FJ, Lewis D, Hershey AD et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics 2012;129(6):e1411–20. [DOI] [PubMed] [Google Scholar]
- 11. Gelfand AA, Goadsby PJ. Treatment of pediatric migraine in the emergency room. Pediatr Neurol 2012;47(4):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaar CR, Gerard JM, Nakanishi AK. The use of a pediatric migraine practice guideline in an emergency department setting. Pediatr Emerg Care 2016;32(7):435–9. [DOI] [PubMed] [Google Scholar]
- 13. Sheridan DC, Laurie A, Pacheco S, Fu R, Hansen ML, Ma OJ, Meckler GD. Relative effectiveness of dopamine antagonists for pediatric migraine in the emergency department. Pediatr Emerg Care 2016. (Epub ahead of print): 1–4. [DOI] [PubMed] [Google Scholar]
- 14. Leung S, Bulloch B, Young C, Yonker M, Hostetler M. Effectiveness of standardized combination therapy for migraine treatment in the pediatric emergency department. Headache 2013;53(3):491–197. [DOI] [PubMed] [Google Scholar]
- 15. Evers S, Rahmann A, Kraemer C et al. Treatment of childhood migraine attacks with oral zolmitriptan and ibuprofen. Neurology 2006;67(3):497–9. [DOI] [PubMed] [Google Scholar]
- 16. Hämäläinen ML, Hoppu K, Valkeila E, Santavuori P. Ibuprofen or acetaminophen for the acute treatment of migraine in children: A double-blind, randomized, placebo-controlled, crossover study. Neurology 1997;48(1):103–7. [DOI] [PubMed] [Google Scholar]
- 17. Gelfand AA, Goadsby PJ. Medication overuse in children and adolescents. Curr Pain Headache Rep 2014;18(7):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta-analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache 2017;57(3):349–62. [DOI] [PubMed] [Google Scholar]
- 19. Minen MT, Torous J, Raynowska J et al. Electronic behavioral interventions for headache: A systematic review. J Headache Pain 2016;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stubberud A, Varkey E, McCrory DC, Pedersen SA, Linde M. Biofeedback as prophylaxis for pediatric migraine: A meta-analysis. Pediatrics 2016;138(2):e20160675. [DOI] [PubMed] [Google Scholar]
- 21. Ernst MM, O’Brien HL, Powers SW. Cognitive-behavioral therapy: How medical providers can increase patient and family openness and access to evidence-based multimodal therapy for pediatric migraine. Headache 2015;55(10):1382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Powers SW, Kashikar-Zuck SM, Allen JR et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: A randomized clinical trial. Jama 2013;310(24):2622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richer LP, Laycock K, Millar K et al. ; Pediatric Emergency Research Canada Emergency Department Migraine Group Treatment of children with migraine in emergency departments: National practice variation study. Pediatrics 2010;126(1):e150–5. [DOI] [PubMed] [Google Scholar]
- 24. FDA Drug Safety Website https://www.fda.gov/Drugs/DrugSafety/ucm549679.htm. (Accessed October 9, 2017).
- 25. Bigal ME, Lipton RB. Overuse of acute migraine medications and migraine chronification. Curr Pain Headache Rep 2009;13(4):301–7. [DOI] [PubMed] [Google Scholar]
- 26. Martin VT, Vij B. Diet and headache: Part 1. Headache 2016;56(9):1543–52. [DOI] [PubMed] [Google Scholar]
- 27. Martin VT, Vij B. Diet and headache: Part 2. Headache 2016;56(9):1553–62. [DOI] [PubMed] [Google Scholar]
- 28. Orr SL, Venkateswaran S. Nutraceuticals in the prophylaxis of pediatric migraine: Evidence-based review and recommendations. Cephalalgia 2014;34(8):568–83. [DOI] [PubMed] [Google Scholar]
- 29. Rajapakse T, Pringsheim T. Nutraceuticals in migraine: A summary of existing guidelines for use. Headache 2016;56(4):808–16. [DOI] [PubMed] [Google Scholar]
- 30. Powers SW, Coffey CS, Chamberlin LA et al. ; CHAMP Investigators Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med 2017;376(2):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rajapakse T, Kirton A.. Brain Stimulation Applications in Pediatric Headache and Pain Disorders. Pediatric Brain Stimulation: Mapping and Modulating the Developing Nervous System. Cambridge, Massachusetts, USA: Elsevier Publications, 2016. [Google Scholar]