Abstract
Background/Aims
In CD34-positive acute myeloid leukaemia (AML), the leukaemia-initiating event likely takes place in the CD34+CD38− cell compartment. CD123 has been shown to be a unique marker of leukaemic stem cells within the CD34+CD38− compartment. The aim of this study was to identify the percentage of CD34+CD38−CD123+ cells in AML blasts, AML CD34+CD38− stem cells, and normal and regenerating bone marrow CD34+CD38− stem cells from non-myeloid malignancies.
Methods
Thirty-eight adult de novo AML patients with intention to treat were enrolled after the application of inclusion criteria from February 2012 to February 2017. The percentage of the CD34+CD38−CD123+ phenotype in the blast population at diagnosis was determined using a CD45-gating strategy and CD34+ backgating by flow cytometry. We studied the CD34+CD38−CD123+ fraction in AML blasts at diagnosis, and its utility as a unique phenotype for minimal residual disease (MRD) of AML patients.
Results
CD123+ cells were present in 97% of AML blasts in patients at diagnosis (median 90%; range 21–99%). CD123+ cells were also present in 97% of the CD34+CD38− compartment (median 0.8164%, range 0.0262–39.7%). Interestingly, CD123 was not present in normal and regenerating CD34+CD38− bone marrow stem cells (range 0.002– 0.067 and 0.004–0.086, respectively).
Conclusion
The CD34+CD38−CD123+ phenotype is present in virtually all AML blasts and it may be used as a unique single phenotype for MRD detection in AML patients.
Keywords: Acute myeloid leukaemia, CD123, CD34+CD38–CD123+ phenotype, Flow cytometry, IL-3 receptor α, Leukaemic stem cells
Introduction
Acute myeloid leukaemia (AML) is a clonal haemopoietic stem cell disorder in which increased proliferation and failure of differentiation in the stem cell compartment results in the accumulation of non-functional immature cells termed myeloblasts. AML accounts for approximately 25% of all leukaemias and is the most common form of acute leukaemia seen in adults [1]. A study of 64 European population-based cancer registries revealed that AML accounts for 43% of all myeloid malignancies estimated to occur annually in Europe [2]. Important advances in therapy and supportive care over the past 3 decades have led to the improved survival of patients with AML [3]. However, despite high complete remission (CR) rates being achieved with modern induction chemotherapy, overall survival (OS) remains at only about 50% for those who achieve CR, or 26% overall [4, 5]. Alarmingly, the average OS rates for men and women in Europe are 15 and 18%, respectively [6]. The outcome for adults with AML depends upon a number of well-defined prognostic markers, such as age, white cell count, cytogenetics, molecular mutations, and whether the AML is de novo or secondary in origin [7, 8, 9].
Multiparameter flow cytometry has many advantages over morphology as it is very sensitive: it can detect 1 cell in a background of 105 or 106 cells, it is relatively cheap and quick, and can detect abnormal cells with high specificity. Hence, it is an important tool for detecting a very low number of abnormal cells at diagnosis and after chemotherapy [10, 11].
Human AML stem cells have been defined as CD34+CD38− cells with a severe combined immunodeficient-repopulating ability, which is a reflection of their capacity to self-renew [12]. In contrast, mature CD34+CD38−/high+ leukaemia cells failed, under appropriate conditions of transplantation, to differentiate and cause an AML malignant transformation [13].
The IL-3 receptor α chain (CD123) has been suggested to be a marker of leukaemic stem cells (LSCs) [14, 15]. These cells are thought to be responsible for initiating and maintaining leukaemic cell growth following chemotherapy and hence give rise to relapse of the disease. It is hypothesised that CD34+CD38−CD123+, which has been identified as a possible putative marker or phenotype of the LSC, could be a useful unique phenotype that can predict an adverse outcome [16]. Previous studies have shown that the LSCs with the CD34+CD38−CD123+ phenotype were preferentially residing in a bone marrow endosteal region niche in a quiescent state untouched by chemotherapy [17, 18]. Enrichment of these chemoresistant cells within this leukaemic compartment supports the theory behind the occurrence of relapse in CR patients [16]. This justifies tracking this particular compartment to assess its clinical utility in AML and minimal residual disease (MRD) monitoring.
The aim of this study was to identify the level of expression of CD123 on total AML blast populations, and LSCs as defined by CD34+CD38− at diagnosis in AML patients. In addition, we also identified the level of expression of CD123 on stem cells as defined by CD34+CD38− in normal bone marrow and regenerating bone marrow with non-myeloid malignancies postchemotherapy to determine the specificity of this phenotype.
Materials and Methods
Samples
Fresh bone marrow samples from adult consecutive, unselected, newly diagnosed, de novo AML patients with intention to treat were enrolled from The Royal Hospital and Sultan Qaboos University Hospital from February 2012 to February 2017. Samples were included in the study after applying the inclusion criteria.
Study Conduct
The study was approved by the Medical Ethics and Scientific Research Committee (MESRC) of the Royal Hospital, the Sultan Qaboos University Medical Research Committee, and the Research and Ethical Review and Approval Committee (RERAC) of the Ministry of Health prior to its initiation. An informed written consent at diagnosis was taken from all patients who were enrolled into the study.
Criteria for Inclusion
For inclusion in this study, a confirmed diagnosis of AML (based on morphology, immunophenotyping, and cytogenetics) and an expression of CD34+ cells were required. Thirty-eight patients were enrolled in the study after application of the inclusion criteria.
The gender, age, WBC count, blasts percentage at diagnosis, clinical cytogenetics, and molecular mutation (FLT3/ITD and NPM1) status were studied for each patient.
Controls (Normal Bone Marrow and Regenerating Bone Marrow)
Normal bone marrow from 5 healthy volunteers and regenerating bone marrow from 5 patients with non-myeloid malignancies treated with chemotherapy were used as controls. The expression of CD123 on CD34+CD38− cells of the normal and regenerating bone marrow was investigated.
Expression of CD123 (IL-3 Receptor) in Total AML CD34+ Blasts and CD34+CD38− AML Stem Cells
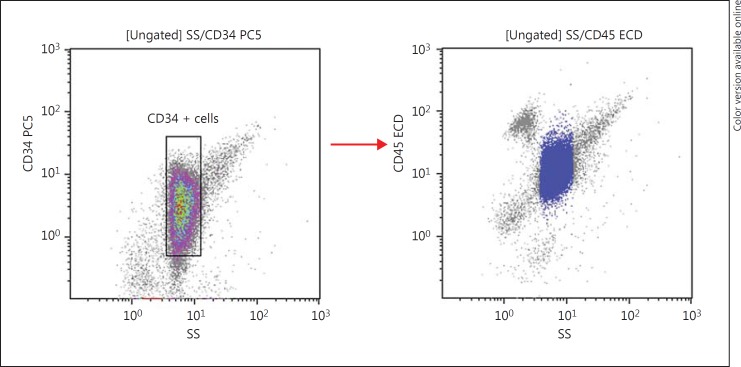
Immunophenotypic analysis was performed on erythrocyte-lysed whole bone marrow samples from the newly diagnosed adult AML patients. Directly conjugated monoclonal antibodies to CD34, CD38, CD45, and CD123 were used to characterise LSC. Blasts were identified by the CD45/SS log gating strategy with backgating strategies using CD34 to better define the blast population (Fig. 1). Appropriate isotype-matched negative controls were used to assess the background fluorescence intensity. The percentage of CD123 was determined on the total AML blast cells expressing CD34+. Using the same gating strategy as shown in Figure 2, the expression of CD123 was determined on the LSC population defined by the phenotype CD34+CD38−.
Fig. 1.
CD34+ backgating strategy to identify the blast population.
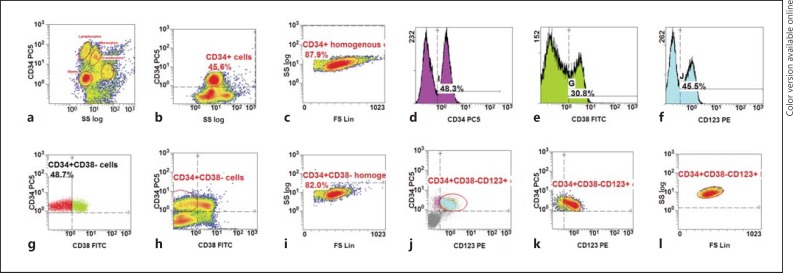
Fig. 2.
Gating strategy in newly diagnosed AML to identify CD34+CD38−CD123+ cells [30]. a Ungated: to identify the blast population based on CD45 dim and low SS log and CD34 backgating. b Ungated: to identify CD34+ cells and the blast population on the CD45/SSC log density plot. c Gated on CD34+ cells: to identify a population homogenous for CD34+ cells. d Ungated: to identify CD34+ cells which resemble the blast populations. e Ungated: to identify a CD38+ population from the total population. f Ungated: to identify a CD123+ population from the total population. g Dot plot: gated on CD34+ homogenous cells. h Density plot: gated on CD34+ homogenous cells. i Density plot: gated on CD34+CD38− cells to identify a population homogenous for the scatter. j Dot plot: gated on CD34+CD38− homogenous cells. k Density plot: gated on CD34+CD38− homogenous cells. l Density plot: gated on CD34+CD38− homogenous cells to identify a population homogenous for the scatter.
Analysis
Cells were analysed on a Cytomics FC500 flow cytometer (Beckman Coulter) and Kaluza Software (version 1.3; Beckman Coulter). Thresholds for positivity were based on isotype controls with a positivity threshold of 20% for all markers. For all samples, not less than 25,000 events were acquired and not less than 20 events in a cluster were considered as positive.
Quality Control
Internal quality control procedures were used to assess the instrument parameters and ensure accurate results. Daily calibration of the flow cytometer was performed for optical laser alignment and optimal hydrodynamic focussing settings. An external quality assurance procedure was also implemented through participation in a performance-monitoring network operated by the Royal College of Pathologists of Australasia Quality Assurance (RCPA) programme. The Royal Hospital is covered under the Canadian Qmentum International Accreditation with the laboratory also being covered by the UKAS Medical Laboratory accreditation (ISO 15189).
Results
The main demographic characteristics of patients (gender, median age, WBC count at diagnosis, cytogenetic risk group, FLT3-ITD and NPM1 mutations) are shown in Table 1. The median age of our cohort was 45 years (range 15–82) and the median WBC count (×109/L) at diagnosis was 29.7 (range 1.2–234).
Table 1.
Characteristics of the 38 AML patients at diagnosis
| Patients, n | 38 |
| Male/female, n | 22/16 |
| Age at diagnosis, years | 45 (15–82) |
| WBC at diagnosis, ×109/L | 29.7 (l.2–234) |
| Blasts at diagnosis by flow cytometry, % | 63.5 (23–95) |
| Cytogenetic risk group | |
| Favourable | 6 (16) |
| Intermediate | 22 (58) |
| Poor | 3 (8) |
| Could not be analysed | 7 (18) |
| FLT3-ITD mutation | |
| Positive | 10 (26.3) |
| Negative | 26 (68.4) |
| Could not be analysed | 2 (5.3) |
| NPM1 mutation | |
| Positive | 2 (5.3) |
| Negative | 27 (81.6) |
| Could not be analysed | 5 (13.1) |
Data are presented as n (%) or median (range).
Expression of CD123 (IL-3 Receptor) in Total AML CD34+ Blasts
CD45 staining and side scatter log properties were used to isolate blasts after optimisation through a backgating strategy using CD34 (the marker found positive on blasts), as shown in Figure 1. The percentage of CD123+ cells were then quantified on the AML blasts. CD123 was found to be expressed in 37 (97%) out of the 38 AML cases analysed. The median expression of CD123 was 90% (range 21–99%).
Expression of CD123 (IL-3 Receptor) in CD34+CD38− AML Stem Cells
The percentage of CD34+CD38−CD123+ cells quantified by sequential gating is shown in Figure 2. CD123 was expressed on CD34+CD38− AML stem cells in 37 (97%) out of the 38 AML patients. The percentage of CD34+CD38−CD123+ cells at diagnosis ranged from 0.0262 to 39.7% (median 0.8164, mean 4.45)
Expression of CD123 (IL-3 Receptor) in CD34+CD38− Normal Bone Marrow and Regenerating Bone Marrow
CD123 was not present in CD34+CD38− normal bone marrow stem cells (range 0.002–0.067%, n = 5). Interestingly, CD123 was also not present in CD34+CD38− regenerating bone marrow stem cells (range 0.004–0.086, n = 5)
Discussion
Relapse of AML starts with leukaemic cell expansion arising from the AML stem cells [19]. In AML, the stem cells are contained within a population of CD34+ and CD38- cells. Since about 50% of patients in CR will eventually experience a relapse [4], a more defined assessment of the quality of CR is important for prognostic purposes. It is thus vital to be able to identify and enumerate the leukaemic cell subpopulation present in the CD34+CD38− fraction that is in a quiescent state and highly resistant to chemotherapy, which is ultimately responsible for the emergence of relapse. The high-affinity IL-3 receptor α, CD123, is a target expressed on the surface of AML LSCs with the capacity to identify and enumerate this leukaemic subpopulation [20].
Our study confirmed the expression of CD123 as being present in virtually all AML blasts (97%). It also demonstrated that CD123 is present in the majority (97%) of AML stem cell CD34+CD38− compartments. The percentage of CD34+CD38−CD123+ cells at diagnosis on AML blasts ranged from 0.02 to 39%. Vergez et al. [16] reported similar levels of CD34+CD38−CD123+ cells using multiparameter flow cytometry with the percentages of leukaemic cells ranging from 0.01 to 67% [16, 21].
Interestingly, our study showed that CD123 is not present in either normal bone marrow or regenerating bone marrow. This ability of CD123 to discriminate between normal cells and LSCs means it is a highly specific and unique marker with the possibility to define MRD using CD34+CD38−CD123+ as a phenotype to detect populations containing LSC [15, 22, 23]. This approach would use a much smaller number of antigens and antigen combinations, resulting in relatively simple data interpretation. It could be a useful prognostic indicator at diagnosis as current chemotherapeutic approaches do not target these cells [24, 25, 26].
Our study also showed that 26% of our AML cohort had the FLT3/ITD mutation. Clinical data suggest that poorer prognosis is related to the presence and sizes of FLT3/ITDs in AML [27, 28, 29]. Nine cases (24%) of the 37 CD123+ AML were FLT3/ITD positive, which reiterates the possibility of an enhanced survival of FLT3/ITD AML LSCs. This was further supported by our recent finding that FLT3/ITD mutations are present at the LSC level, which also showed that the oncogenic events of FLT3/ITD occurred at a cell stage possessing the IL-3 receptor α (CD123) [30]. This correlation forms a basis for CD123-targeted therapies in AML with intracellular FLT3 inhibitors [31].
A number of studies suggest that targeting of the CD123/IL-3 receptor α may be a novel and promising treatment approach in patients with CD123+ AML [32, 33, 34]. This concept is based on the notion that myeloblasts express CD123 in most patients with AML, as shown in our study. Our results demonstrate the utility of CD34+CD38−CD123+ cells, which may be used as the basis of risk-stratified AML clinical trials. Previous studies have shown that high levels of CD34+CD38− stem cells at diagnosis, as well as high levels of CD34+CD38+CD123+ cells, are predictive of high MRD and an adverse outcome in terms of relapse-free survival and OS [16, 35]. Recent studies have shown anti-CD123 antibodies as targets against AML LSCs. Adams et al. [36] showed that IMGN632, a CD123-targeting antibody drug conjugate, was highly potent in vitro against CD123-expressing human AML cell lines with prolonged survival in immunodeficient mice. A phase 1 study of CSL362, another anti-CD123 antibody, showed the potency and specific targeting of normal cells expressing high CD123 in AML patients in CR at high risk for early relapse [37]. These promising CD123-targeing studies emphasise the importance of evaluating the CD34+CD38−CD123+ fraction in AML patients. Considering the use of CD123-targeted drugs in AML, it is of particular importance to identify that these cells express leukaemia-specific molecular abnormalities, such as FLT3 mutations.
Eradication of malignant stem cells can probably be achieved in some patients using conventional chemotherapy through the application of repetitive cycles of consolidation therapy [38]. However, given that LSCs are contained within the CD34+CD38−CD123+ population in AML and the apparent difficulties in eradicating malignant cells from the patient, it will be necessary to develop therapies that specifically target the malignant stem cells of this phenotype after reaching CR. The possibility to detect and characterise stem cells will help in reaching this goal.
In the future, it would be of great interest to study the frequency of CD34+CD38−CD123+ cells during follow-up as these cells may represent the highly resistant cells to chemotherapy and might be the cells that cause regrowth of leukaemia and thus relapse of the disease. In principle, this would allow us to correlate the LSC frequency at diagnosis with LSC frequency in CR after chemotherapy. It would be of value to study if the prognostic impact of the MRD stem cell frequency might improve the already strong impact of total MRD frequency in leukaemia-associated phenotypes on outcome. Apart from the leukaemia-associated phenotype expression, the LSC selective expression of CD123 and of the recently described CLL-1 [39, 40] should be very helpful in this respect.
Conclusion
Our study shows that the CD34+CD38−CD123+ phenotype is present in virtually all AML blasts and it may be used as a unique single phenotype for MRD detection in AML patients. MRD will significantly improve the evaluation of the prognosis of patients with AML and ultimately play a major role as a stratification parameter to guide the risk-adapted therapy of the disease. With the functional and phenotypic properties of LSCs being further explored, these cells appear to be the most important target cell population in the context of curative therapies [41, 42]. Therefore, the potent anti-leukaemic effects of “stem cell-targeted” drug therapy is a very promising approach for the future. Whether these strategies will help in the eradication of AML clones and the management of minimal residual AML remains to be determined in future clinical trials.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
This work was supported by The Research Council (TRC), Oman (grant No. 47).
Acknowledgements
The authors would like to acknowledge Sabria Al-Hashmi and Anil Pathare for their assistance in procuring samples for the study. The authors would also like to thank Raiya Al-Busaidi, Rabab Al-Lawati, and Sahimah Al-Mamari for their technical support.
References
- 1.Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099–2107. doi: 10.1002/cncr.22233. [DOI] [PubMed] [Google Scholar]
- 2.Visser O, Trama A, Maynadié M, Stiller C, Marcos-Gragera R, De Angelis R, Mallone S, Tereanu C, Allemani C, Ricardi U. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012;48:3257–3266. doi: 10.1016/j.ejca.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Percival M-EM, Tao L, Medeiros BC, Clarke CA. Improvements in the early death rate in 9,380 acute myeloid leukemia patients following initial therapy: a SEER database analysis. Cancer. 2015;121:2004. doi: 10.1002/cncr.29319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Rowe JM, Radich J, Dick JE. Acute myeloid leukemia. Hematol Am Soc Hematol Educ Program. 2001;2001:62–86. doi: 10.1182/asheducation-2001.1.62. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society . Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 6.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5 - a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 7.De Santis GC, Benicio MT, Oliveira L, Falcão RP, Rego EM. Genetic mutations in patients with acute myeloid leukemia and leukostasis. Acta Haematol. 2013;130:95–97. doi: 10.1159/000346442. [DOI] [PubMed] [Google Scholar]
- 8.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Löffler H, Sauerland CM, Serve H, Büchner T. Analysis of FLT3 length mutations in 1,003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Al-Mawali A, Gillis D, Lewis I. The role of multiparameter flow cytometry for detection of minimal residual disease in acute myeloid leukemia. Am J Clin Pathol. 2009;131:16–26. doi: 10.1309/AJCP5TSD3DZXFLCX. [DOI] [PubMed] [Google Scholar]
- 11.Al-Mawali A, Gillis D, Hissaria P, Lewis I. Incidence, sensitivity, and specificity of leukemia-associated phenotypes in acute myeloid leukemia using specific five-color multiparameter flow cytometry. Am J Clin Pathol. 2008;129:934–945. doi: 10.1309/FY0UMAMM91VPMR2W. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML) Blood. 2003;101:3142–3149. doi: 10.1182/blood-2002-10-3062. [DOI] [PubMed] [Google Scholar]
- 13.Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut J-A, Olive D. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- 14.Jordan C, Upchurch D, Szilvassy S, Guzman M, Howard D, Pettigrew A, Meyerrose T, Rossi R, Grimes B, Rizzieri D. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, Guthridge MA, Thomas D, Barry EF, Boyd A. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Vergez F, Green AS, Tamburini J, Sarry J-E, Gaillard B, Cornillet-Lefebvre P, Pannetier M, Neyret A, Chapuis N, Ifrah N. High levels of CD34+ CD38low/− CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucémies Aiguës et Maladies du Sang (GOELAMS) study. Haematologica. 2011;96:1792–1798. doi: 10.3324/haematol.2011.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 19.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Mawali A. Leukemic stem cells shows the way for novel target of acute myeloid leukemia therapy. J Stem Cell Res Ther. 2013;3:151. [Google Scholar]
- 21.Zhao M, Zhu H, Rajbhandary Sajin XX, Deng Q. Clinical significance of leukemia stem cells immunophenotype expression in patients with acute leukemia. Life Sci J. 2013;10:2543–2548. [Google Scholar]
- 22.Campana D, Coustan-Smith E. Minimal residual disease studies by flow cytometry in acute leukemia. Acta Haematol. 2004;112:8–15. doi: 10.1159/000077554. [DOI] [PubMed] [Google Scholar]
- 23.Guzman ML, Allan JN. Concise review: leukemia stem cells in personalized medicine. Stem Cells. 2014;32:844–851. doi: 10.1002/stem.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollyea DA, Gutman JA, Gore L, Smith CA, Jordan CT. Targeting acute myeloid leukemia stem cells: a review and principles for the development of clinical trials. Haematologica. 2014;99:1277–1284. doi: 10.3324/haematol.2013.085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal S, Rodriguez-Bravo V, Galsky M, Cordon-Cardo C, Domingo-Domenech J. Targeting cancer stem cells to suppress acquired chemotherapy resistance. Oncogene. 2014;33:4451–4463. doi: 10.1038/onc.2013.411. [DOI] [PubMed] [Google Scholar]
- 27.Rombouts W, Blokland I, Löwenberg B, Ploemacher R. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia. 2000;14:675. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 28.Stirewalt DL, Kopecky KJ, Meshinchi S, Engel JH, Pogosova-Agadjanyan EL, Linsley J, Slovak ML, Willman CL, Radich JP. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–3726. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Mawali A, Gillis D, Lewis I. Characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplication in the FLT3 gene. Oman Med J. 2013;28:432–440. doi: 10.5001/omj.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Mawali A, Gillis D, Lewis I. Immunoprofiling of leukemic stem cells CD34+/CD38−/CD123+ delineate FLT3/ITD-positive clones. J Hematol Oncol. 2016;9:61. doi: 10.1186/s13045-016-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su L, Gao S-J, Li W, Tan Y-H, Cui J-W, Han W. The surface molecular signature of leukemic cells is associated with NPM1 mutations and FLT3-ITD in patients with de novo acute myeloid leukemia. Acta Haematol. 2014;131:148–152. doi: 10.1159/000353663. [DOI] [PubMed] [Google Scholar]
- 32.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 33.Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, Brunet S, Sierra J. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–1269. [PubMed] [Google Scholar]
- 34.Moretti S, Lanza F, Dabusti M, Tieghi A, Campioni D, Dominici M, Castoldi GL. CD123 (interleukin 3 receptor alpha chain) J Biol Regul Homeost Agents. 2001;15:98–100. [PubMed] [Google Scholar]
- 35.van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, van der Pol MA, Waisfisz Q, Ossenkoppele GJ, Schuurhuis GJ. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 36.Adams S, Wilhelm A, Harvey L, Bai C, Yoder N, Kovtun Y, Chittenden T, Pinkas J. IMGN632: a CD123-targeting antibody-drug conjugate (ADC) with a novel DNA-alkylating payload, is highly active and prolongs survival in acute myeloid leukemia (AML) xenograft models. Blood. 2016;128:2832. [Google Scholar]
- 37.Smith BD, Roboz GJ, Walter RB, Altman JK, Ferguson A, Curcio TJ, Orlowski KF, Garrett L, Busfield SJ, Barnden M. First-in man, phase 1 study of CSL362 (anti-IL3Rα/anti-CD123 monoclonal antibody) in patients with CD123+ acute myeloid leukemia (AML) in CR at high risk for early relapse. Blood. 2014;124:120. [Google Scholar]
- 38.Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E., 3rd Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 39.Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, Jongeneelen MA, Visser TJ, Bijl N, Geuijen CA, Marissen WE, Radosevic K, Throsby M, Schuurhuis GJ, Ossenkoppele GJ, de Kruif J, Goudsmit J, Kruisbeek AM. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 40.van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Walsum MS, Zweegman S, Ossenkoppele GJ, Jan Schuurhuis G. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 41.Han L, Jorgensen JL, Brooks C, Shi C, Zhang Q, González GMN, Cavazos A, Pan R, Mu H, Wang S. Antileukemia efficacy and mechanisms of action of SL-101, a novel anti-CD123 antibody-conjugate, in acute myeloid leukemia. Clin Cancer Res. 2017;23:3385–3395. doi: 10.1158/1078-0432.CCR-16-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su R, Chen M. CD123 is a useful marker for prediction of clinical outcome and risk stratification for prognosis in leukemia patients. Am J Clin Pathol. 2015;144:A139–A139. [Google Scholar]