Abstract
Adverse outcome pathways are designed to provide a clear-cut mechanistic representation of critical toxicological effects that propagate over different layers of biological organization from the initial interaction of a chemical with a molecular target to an adverse outcome at the individual or population level. Adverse outcome pathways are currently gaining momentum, especially in view of their many potential applications as pragmatic tools in the fields of human toxicology, ecotoxicology and risk assessment. A number of guidance documents, issued by the Organization for Economic Cooperation and Development, as well as landmark papers, outlining best practices to develop, assess and use adverse outcome pathways, have been published in the last few years. The present paper provides a synopsis of the main principles related to the adverse outcome pathway framework for the toxicologist less familiar with this area, followed by two case studies relevant for human toxicology and ecotoxicology.
Keywords: adverse outcome pathway, risk assessment, toxicology, ecotoxicology
1. Introduction
Predictive toxicology based upon mechanistic information has become a critical aspect of chemical risk assessment in the last two decades. A major step in this direction came with the introduction of the mode-of-action concept in 2001, which encompasses a series of key events (KEs) along a biological pathway from the initial chemical interaction to the adverse outcome (AO) (Meek et al., 2014a and 2014b; Sonich-Mullin et al., 2001). The mode-of-action concept was first used by the US Environmental Protection Agency (EPA) and adopted by others in the cancer field (Boobis et al., 2006; Dellarco and Wiltse, 1998; Preston and Williams, 2005; Wiltse and Dellarco, 2000), but seemed equally exploitable for non-cancer endpoints (Boobis et al., 2008). Another milestone was the seminal report published by the US National Academy of Science in 2007, presenting a vision on toxicology in the twenty-first century and placing both in vitro toxicology and toxicity pathways in the foreground. These toxicity pathways denote cellular pathways that, when disturbed, can lead to adverse health effects (NRC, 2007). Toxicity pathways align to a large extent with adverse outcome pathways (AOPs), introduced in 2010 in the field of ecotoxicology (Ankley et al., 2010). An AOP refers to a conceptual construct that portrays existing knowledge concerning the linkage between a direct molecular initiating event (MIE) and an AO at a biological level of organization relevant to risk assessment. Although conceptually very similar, the scope of an AOP is broader compared to the mode-of-action, as it can go up to the population and even ecological level. Furthermore, while the mode-of-action tends to be chemical-specific and takes into account kinetic aspects such as metabolism, AOPs are chemical-agnostic in that they describe a toxicological process from a purely dynamic, biological perspective. Thus, an AOP can be ultimately associated with any chemical that is bioavailable at the relevant site of action and which has the specific properties to activate the associated MIE (Becker et al., 2015; Burden et al., 2015; Edwards et al., 2016; Perkins et al., 2015; Villeneuve et al., 2014a).
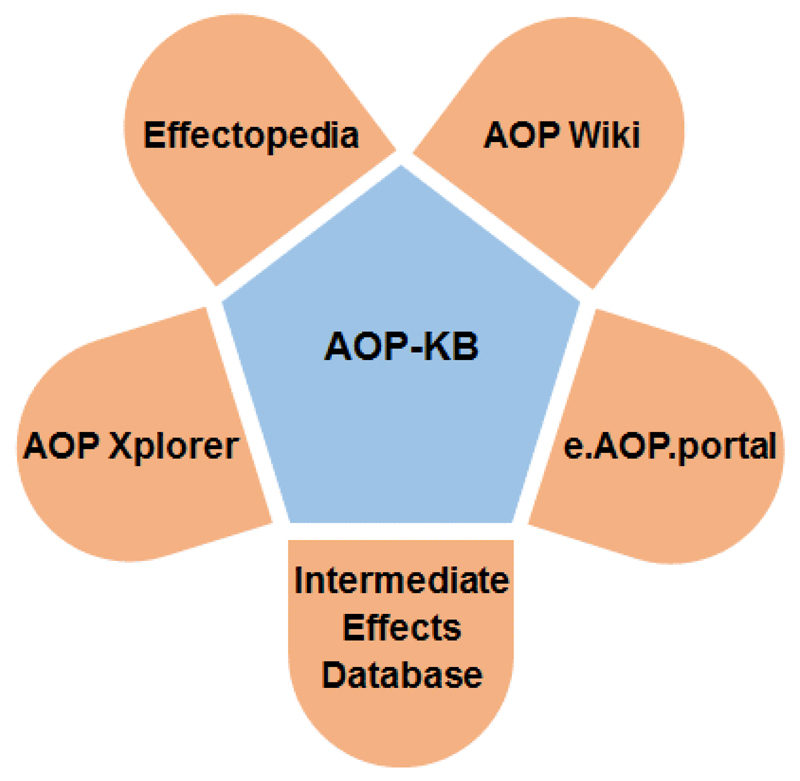
Because of the increasing use of AOPs, a clear need to formalize the AOP development and evaluation process emerged. As a result, the Organization for Economic Cooperation and Development (OECD) initiated an international AOP development program in 2012, followed by the publication of a guidance document on the development and assessment of AOPs (OECD, 2013). In 2014, the OECD, together with the Joint Research Centre of the European Commission (JRC), the US EPA and the US Army Engineer Research and Development Centre, launched the AOP knowledge base (AOP-KB). The AOP-KB consists of 5 modules, namely the e.AOP.Portal, AOP Xplorer, Effectopedia, the Intermediate Effects Database and the AOP Wiki (Figure 1). The AOP Wiki currently is the most actively maintained and most densely populated AOP-KB module, and provides an open-source interface that serves as a central repository for qualitative AOPs and as such facilitates sharing of AOP knowledge and collaborative AOP development. The e.AOP.Portal is the main entry point for the AOP-KB and provides a search engine that allows querying both AOPs and KEs. The AOP Xplorer, still under development, is a computational tool that enables automated graphical representation of AOPs and their networks. Effectopedia, currently available as a standalone application only, but expected to be released as an online tool in the near future, assembles data on quantitative relationships underpinning KERs. Finally, the Intermediate Effects Database will capture intermediate effect data from toxicity studies following the OECD Harmonized Templates format, serving as empirical evidence to support AOP development. Furthermore, the OECD published a user's handbook as a supplement to the guidance document on AOP development, which provides in-depth information on AOP development, including practical instructions on how to build and evaluate AOPs (OECD, 2016). An AOP developed within a project of the AOP work program at the OECD undergoes a rigorous and transparent internal and external review process. If successfully completed, this ends with endorsement and publication in the OECD Series on AOPs (Delrue et al., 2016). At present, the AOP Wiki contains about 200 AOPs for a plethora of human and ecotoxicological endpoints and identification of more than 250 stressors triggering one or more of the MIEs in the AOP Wiki. Aligned with these developments, two landmark papers were published in 2014, outlining strategies, principles and best practices related to AOP development (Villeneuve et al., 2014a and 2014b). Several other excellent review manuscripts have been published in the last few years, each focusing on a particular aspect of AOP development, evaluation and application (Becker et al., 2015; Burden et al., 2015; Delrue et al., 2016; Edwards et al., 2016; Perkins et al., 2015).
Figure 1.
Modules of the AOP-KB. The e.AOP.portal can be reached at http://aopkb.org, the AOP Wiki at http://aopwiki.org, Effectopedia at http://effectopedia.org and the AOP Xplorer at http://datasciburgoon.github.io/aopxplorer. The OECD “Harmonized Template 201: Intermediate effects” can be found at https://www.oecd.org/ehs/templates/harmonised-templates-intermediate-effects.htm.
The present document intends to provide a synoptic “helicopter view” of the AOP field for the toxicologist less familiar with this area. The different elements of AOP development, assessment and application are subsequently illustrated in two case studies pertinent to the fields of human toxicology and ecotoxicology.
2. AOP development
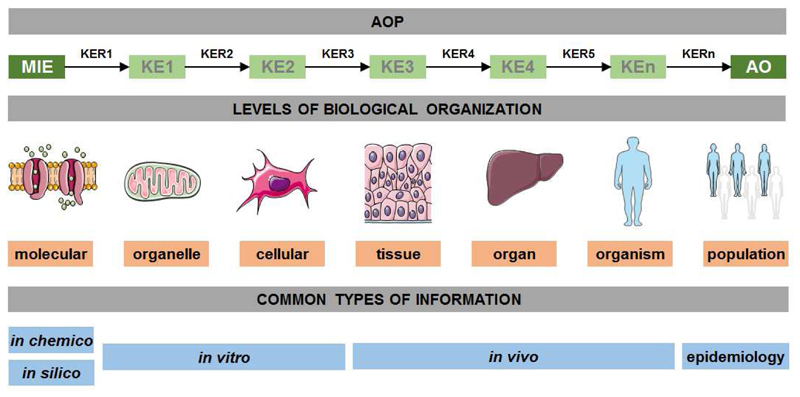
Each AOP comprises two fundamental modular components, namely key events (KEs) and key event relationships (KERs), which link together in a causal chain that spans from the molecular domain all the way up to the level of the organism or even population (Figure 2). Modularity hereby refers to the aim of breaking down a complex toxicological process into discrete tractable information elements (i.e. KEs and KERs) to make it easier to understand and describe, and to allow the re-use of previously described KEs or KERs in other AOPs. A KE represents a measurable change in a biological state that is essential, but not necessarily sufficient, for progression from the MIE to the AO. The MIE and AO are two specialized KE types, but are otherwise treated similarly in the AOP framework. The MIE occurs by definition at the molecular level and indicates the initial point of chemical-biological interaction within the organism. The AO, situated typically at the organ level or higher, indicates a change in morphology or physiology of an organism or system that results in impairment of the functional capacity or the capacity to compensate for stress. Adversity is typically considered as relevant to act upon from a risk assessment or risk management perspective, yet could in principle be any effect that is considered adverse within a given research or application context. A KER defines a causal relationship between a pair of KEs, establishing one as upstream and one as downstream. It provides the scientifically plausible and evidence-based foundation for extrapolation from an upstream cause to a downstream effect, and thus for using KE information as indicators of adverse effects. Furthermore, a KER may reflect linkages between a pair of KEs that are either adjacent or non-adjacent in an AOP allowing the possibility to capture parallel and interdependent processes within a single AOP (OECD, 2013 and 2016; Villeneuve et al., 2014a and 2014b).
Figure 2.
Generic AOP structure, in this case applied to human toxicology, spread over different levels of biological organization and fed by different types of information.
As such, five core principles have been defined to guide AOP development (Villeneuve et al., 2014a; EPA 2017), namely (i) AOPs are not chemical-specific, (ii) AOPs are modular, (iii) individual AOPs are the pragmatic units of development and evaluation, (iv) AOP networks are the functional units of prediction for most real-world applications, and (v) AOPs are living frameworks. While the first 2 principles deal with the basic components of AOPs, the last 3 principles pertain more generally to the development and application of the construct (Edwards et al., 2016). Different types of information can be used during AOP development. Characterization of the MIE generally relies on in chemico, in silico or in vitro data. Early KEs at the organelle and cellular level are typically supported by in vitro or in vivo testing outcomes, while intermediate and late KEs at the tissue, organ and organism level routinely depend on in vivo experimentation results. When incorporating population level effects, AOP development may necessitate epidemiological or population biology information (Figure 2) (Delrue et al., 2016; Edwards et al., 2016). Kinetics information, including data on absorption, distribution, metabolism and excretion (ADME), is not considered in the AOP development process, as AOPs are developed to be chemical-agnostic. ADME is intrinsically chemical-specific and although not captured in AOP development, kinetic data are essential in the application phase of AOPs for associating external exposure with internal exposure information at the site of the molecular target (OECD, 2013), a key area where AOPs can be used in problem formulation at the early stages of an assessment.
AOPs can be developed by following a number of strategies. In top-down, middle-out and bottom-up approaches, AOP development starts from an MIE, KE or AO, respectively. Case study strategies begin with an AOP based on one or a few model chemicals, which are subsequently generalized to other chemicals and/or stressors. AOP development by analogy defines an AOP in one organism and intends extrapolation to other species. AOP development from data-mining uses high-throughput and high-content data to identify KEs and infer the linkages between them (Bell et al, 2016; Oki and Edwards, 2016; Oki et al., 2016). These different AOP development strategies can be combined and their selection is determined, at least in part, by the intended AOP application (Villeneuve et al., 2014a) and in most cases to a large extent by the availability of existing data. The AOP development process is a continuous activity and is about gradually building an AOP from a hypothesized set of KEs and KERs and providing the evidence base to support it (Edwards et al., 2016; Perkins et al. 2015; Villeneuve et al., 2014a). Initial steps may rely more on rather a loose pooling of general toxicological knowledge or statistical inference derived from datasets. As development progresses, the endeavour shifts to refinement of KEs and KERs definitions and a more formal, systematic and rigorous assembly of evidence underpinning the AOP. Such evidence fuses consideration of biological plausibility with supporting empirical data and can be drawn from the scientific literature or generated prospectively in the case of knowledge or data gaps. Moreover, evidence is not only presented within an AOP, but is also assessed in a weight-of-evidence process that serves to convey the confidence, which an eventual user of an AOP might attribute to it. Finally, although an AOP is typically described in qualitative language, aspects, such as the causality described in a KER, can be quantified in terms of, for example, a mathematical response-response relationship. Although highly desirable, quantification of AOPs is often severely hindered by the lack of relevant data and the difficulty and cost of generating them.
3. AOP assessment
Evaluation of newly developed AOPs includes consideration of the so-called tailored Bradford-Hill criteria. The Bradford-Hill criteria have been initially introduced to determine causality of associations observed in epidemiological studies (Hill, 1965). In the last few years, they have been adopted to assess AOPs, albeit in a more tailored format. In rank order, these tailored Bradford-Hill considerations include biological plausibility, essentiality and empirical support (Table 1). While the former and the latter are considered for each KER individually, essentiality of the KEs is scrutinized in the context of the overall AOP. Each of these tailored Bradford-Hill considerations is subjected to weight-of-evidence analysis, whereby confidence should be judged as high/strong, moderate or low/weak for each of the KEs, KERs and the AOP as such, based on the availability of documentation and/or empirical support (Becker et al., 2015). The purpose of this weight-of-evidence analysis is to transparently document the certainty and uncertainty existing in specific lines of evidence that support the overall MIE-to-AO relationship (Collier et al., 2016).
Table 1.
Tailored Bradford-Hill criteria for AOP assessment (Becker et al., 2015; adapted from US EPA’s AOP Research Brief (EPA, 2017)).
| Criterion | Driving questions |
|---|---|
| Biological plausibility. | Is there a mechanistic/systemic relationship between KEupstream and KEdownstream consistent with established biological knowledge? |
| Essentiality. | Are the KEdownstream and/or AO prevented if KEupstream is blocked? |
| Empirical support. | - Does the empirical evidence support that a change in KEupstream leads to an appropriate change in KEdownstream? - Does KEupstream occur at lower doses and earlier time points than KEdownstream and is the incidence of KEupstream greater than that for the KEdownstream? - Are there inconsistencies in empirical support across taxa, species and stressors that do not align with an expected pattern for the hypothesized AOP? |
4. Applications of AOPs
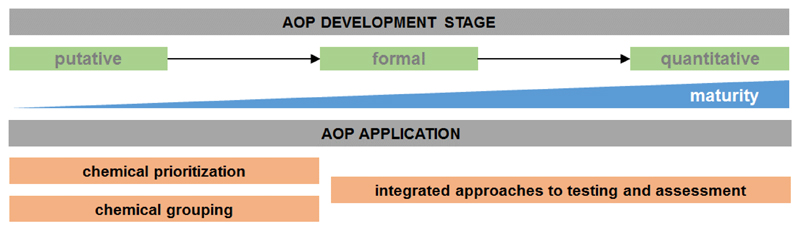
AOPs were intended specifically to support regulatory decision-making based on the desire to make effective use of mechanistic data, particularly novel data streams that can be generated more rapidly and cost-effectively in a high-throughput format, rather than relying only on apical-outcome data traditionally measured in whole-organism guideline toxicity tests (Burden et al., 2015; Dean et al., 2017; Delrue et al., 2016; Edwards et al., 2016; Villeneuve et al, 2014a). The specific application of an AOP is usually dictated by the amount of available experimental and observational data and the AOP’s degree of maturity, including the level of quantitative understanding of the AOP (Figure 3), but is constrained by its taxonomic, sex and life-stage applicability domain.
Figure 3.
AOP development process and potential applications in toxicology and risk assessment. There are no clear boundaries between the different stages of development or levels of completeness (i.e. putative AOP, formal AOP and quantitative AOP). These terms rather reflect a continuum of development and in most cases it is not possible to assign specific applications to a specific category. Instead, the applicability of a given AOP most often depends on a case by case determination of fit-for-purpose.
AOPs can serve as the basis for generating integrated approaches to testing and assessment (IATA). IATA is a pragmatic approach that exploits and weighs existing information, including human data and exposure information, alternative methodologies, such as in chemico and in vitro assays, and tailored strategies for the purpose of chemical evaluation with applications in risk assessment (Patlewicz et al., 2015; Tollefsen et al., 2014). While IATAs provide a platform for data integration and a means for targeted testing for a specific purpose, it is not necessarily framed by a mechanistic rationale. AOPs could be used to provide this mechanistic basis and thus to identify data gaps or to contextualize a diverse universe of existing data (Delrue et al, 2016, Tollefsen et al., 2014).
Another AOP application includes the development of chemical categories based on biological responses. A chemical category is defined as a group of chemicals whose physico-chemical and human health properties are likely to be similar or to follow a regular pattern. The similarities are typically based on common functional features. The next step is to substantiate the tentative group with experimental data and non-testing approaches, such as quantitative structure-activity relationship (QSAR) methodologies. Once the chemical category is fully established, it can be used for data gap filling strategies, such as read-across techniques that apply relevant information from analogous substances to predict the toxicological properties of a target substance (OECD, 2013; Patlewicz et al., 2015). In this respect, the OECD provides a QSAR Toolbox, which is a free software package for supporting read-across based on chemical categories (https://www.qsartoolbox.org/). A main feature of the OECD QSAR Toolbox is the definition of profilers, which are structural alerts associated with the induction of specific MIEs (Delrue et al., 2016).
AOPs may also facilitate prioritization of chemicals for assessment. In this process, substances are screened for their potential to trigger a specific and measurable biological response, which in an AOP context could be related to one or several MIEs, KEs and/or AOs. Substances identified as presenting an unreasonable risk to cause an AO are subsequently ranked according to potency, whereby the most potent substances receive highest priority to undergo more detailed testing and/or evaluation (Burden et al., 2015; OECD, 2013a; Patlewicz et al., 2015).
Several other applications for AOPs have been described, including the development or refinement of methods to test specific KEs, the development of alternatives to animal testing as well as the classification and labelling of chemicals based on their potency to activate an AOP (OECD, 2013; Patlewicz et al., 2015, Villeneuve et al., 2014c).
5. AOP case studies
Two case studies will be discussed, namely (i) the AOP from covalent protein binding leading to skin sensitization in humans as an example of an AOP in human toxicology, and (ii) the AOP from aromatase inhibition leading to reproductive dysfunction in fish as an example of an AOP in ecotoxicology. These case studies serve to illustrate the general principles laid out in the previous sections, and to specifically show how AOPs can be used to develop and provide confidence in alternative assays to traditional animal toxicity testing by describing the link between a MIE, which can be measured using an alternative assay, and a relevant AO.
The human apical toxicological endpoint most elaborated in terms of the AOP concept is probably skin sensitization (AOP 40 in the AOP Wiki). Skin sensitization is the process that drives allergic contact dermatitis, which accounts for 10-15% of all occupational diseases worldwide and that imposes a considerable burden on healthcare systems and economy (Basketter et al., 2015). Indeed, many chemicals, including detergents, preservatives and fragrances in household and personal care products as well as active ingredients and impurities in synthetic, industrial or pharmaceutical products, may act as skin sensitizers. Therefore, skin sensitization is an important toxicological endpoint routinely included in contemporary chemical safety assessment (Wang et al., 2017). This has urged the need for developing alternative methods to reduce the need for animal tests.
In ecotoxicology, a number of AOPs for endocrine disruption are currently under development in order to find alternatives for the large numbers of fish tests that are currently needed to assess endocrine disruption potential of chemicals in the aquatic environment. For example, AOPs for inhibition of thyroperoxidase and deiodinases, enzymes involved in the activation and synthesis of thyroid hormones, are under development, and in vitro assays based on these AOPs are being validated to screen chemicals for their ability to disrupt the thyroid axis in fish (Paul et al., 2013; Paul et al., 2014; Nelson et al., 2016; Stinckens et al., 2016). However, the main category of AOPs related to endocrine disruption currently being developed are AOPs initiated by perturbations of the steroid hormone system, including estrogen receptor agonism, estrogen receptor antagonism and androgen receptor agonism leading to reproductive dysfunction in fish (i.e. AOP 23, 25, 29 and 30 in the AOP Wiki). The AOP for aromatase inhibition leading to reproductive dysfunction in female fish is the most advanced AOP within this category today (AOP 25 in the AOP Wiki) with a high level of quantitative understanding, and hence will be discussed as a case study.
5.1. AOP from covalent protein binding leading to skin sensitization in humans
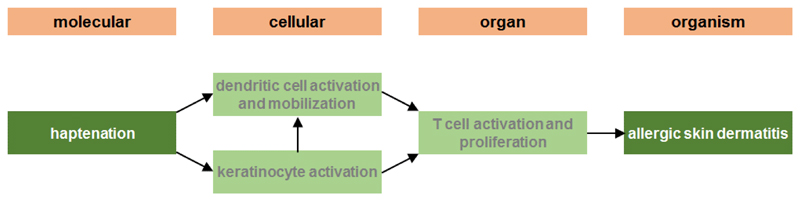
Skin sensitization comprises two stages. In the first stage, called the sensitization or induction phase, a first contact of the skin with the chemical takes place, during which an immunological memory is generated. In the second stage, called the elicitation or challenge phase and that occurs upon next exposures of the skin to the compound, the actual clinical effects become manifest (Urbisch et al., 2015). In 2012, the OECD endorsed and subsequently published an AOP describing the pathways underlying skin sensitization (Figure 4) (OECD, 2014). The MIE takes place following penetration of the chemical into the skin. The parent chemical or its metabolite then forms a stable complex with carrier proteins in the skin, yielding a product that is immunogenic, a process called haptenation. The hapten-protein complex further reacts with keratinocytes, the main cell population in the epidermis. This first KE is characterized by the activation of the inflammatory machinery and oxidative defence, such as triggered by the Kelch-like ECH-associates protein 1 (Keap1)/nuclear erythroid 2-related factor (Nrf2) signalling cascade. Simultaneously, the hapten-protein complex activates a second KE, namely the activation and maturation of dendritic cells. Activated dendritic cells subsequently move to the lymph nodes where they present the hapten to naïve T cells. This constitutes the third KE, implying proliferation of hapten-specific T cells and the generation of antigen-specific memory T cells, some that circulate in the body. Upon next contact of the skin with the chemical, the challenge phase, the hapten-protein complex is again formed and taken up by dendritic cells, but also by antigen-presenting cells. The circulating antigen-specific memory T cells then start to secrete cytokines that induce the release of inflammatory mediators mobilizing cytotoxic T cells and other inflammatory cells. These cells migrate to the epidermis and elicit an inflammatory response, the final AO, associated with red rash, blisters, itchy and burning skin (OECD, 2012).
Figure 4.
AOP from covalent protein binding leading to skin sensitization in human (https://aopwiki.org/aops/40).
The AOP from covalent protein binding leading to skin sensitization was the first to have been endorsed by the OECD after a stringent review process and since then 5 more have also been endorsed and published (Delrue et al., 2016). This AOP is also among the first to be applied as a quantitative AOP. The latter includes mathematical models for dose and duration linking KEs, such as haptenation and T cell activation, which are required to support risk assessment applications (Conolly et al., 2017; Maxwell et al., 2014). Furthermore, the skin sensitization AOP is included in the third version of the OECD QSAR Toolbox released in 2012, where specific profilers are defined for each of the MIE, KEs and the AO (http://qsartoolbox.org), and in integrated regulatory toxicity testing strategies, such as IATA, where a battery of methods are available to test the MIE, KEs, and AO (Ezendam et al., 2016; Patlewicz et al., 2014). The MIE can be studied by means of the in chemico direct peptide reactivity assay (DPRA) that measures the depletion of a peptide with lysine and another one with cysteine as key reactive sites after incubation with an excess of the test chemical, using quantitative high-pressure liquid chromatography (Gerberick et al., 2004). Regarding the first KE (i.e. keratinocyte activation), reactive chemicals bind to Keap1 leading to Keap1 dissociation from Nrf2. Released Nrf2 then translocates to the nucleus to trigger the antioxidant response element (ARE) in the promotor region of target genes. The KeratinoSens and LuSens tests are two in vitro luciferase reporter gene assays that measure ARE activation following cell exposure to test chemicals (Emter et al., 2010; Ramirez et al., 2014). The second KE (i.e. dendritic cell activation) can be detected using the myeloid U937 skin sensitization test (MUSST). In this in vitro assay, myeloid U937 cells are incubated with the test chemical followed by flow cytometry-based measurement of CD86 expression, a co-stimulatory molecule indicative of dendritic cell activation (Python et al., 2007). A similar in vitro method is the human cell line activation test (hCLAT) that uses THP-1 monocytic cells and measures CD54 expression, a marker of dendritic cell activation, in addition to CD86 (Ashikaga et al., 2006; Sakaguchi et al., 2006). The third KE (i.e. T cell activation and proliferation) may be evaluated by means of the in vivo local lymph node assay (LLNA). In the LLNA, the test chemical is applied onto the skin of mice followed by injection of 3H-methyl-thymidine. Thereafter, the lymph nodes behind the ears are removed and T cell proliferation is inferred from scintillation counting of 3H-methyl-thymidine incorporation into newly synthesized DNA (Kimber et al., 1986). The AO can be tested in vivo using the Guinea pig maximization test (GPMT), in which the test chemical is injected together with an adjuvant. This is followed by skin damage, application of the test chemical to the skin and evaluation of the skin condition (Magnusson and Kligmann, 1969). A number of these methods, namely the DPRA assay, the KeratinoSens test, the hCLAT test, the LLNA and GPMT, have been formally embedded in OECD test guidelines. Several other non-animal methods are currently being developed and/or are under validation by the European Unio Reference Laboratory for Alternatives to Animal Testing (Ezendam et al., 2016). A significant milestone for mechanistic-based approaches to toxicological hazard assessment was achieved during the autumn of 2016, when the REACH (i.e. Registration, Evaluation, Authorization and Restriction of Chemicals) information requirements for skin sensitisation were changed. Now, provision of information relating to KEs of the skin sensitisation AOP using non-animal tests is the default route.
5.2. AOP from aromatase inhibition leading to reproductive dysfunction in fish
A number of chemicals entering the environment, including pesticides and drugs used to treat breast cancer, have the potential to inhibit aromatase, the enzyme responsible for converting testosterone to 17-beta-estradiol (E2) (Hinfray et al., 2006). Aromatase inhibition leads to reduced synthesis of E2 in the ovaries of fish, in turn leading to reduced plasma E2 levels (Figure 5) (Ankley et al., 2009). Reduced E2 levels result in decreased vitellogenin (VTG) synthesis by the liver, since this gene is under estrogenic control (Villeneuve et al., 2009). In oviparous vertebrates, VTG functions as an egg yolk precursor glycoprotein, essential for oocyte development and larval survival. Reduced plasma VTG levels therefore result in impaired oocyte growth and development, reduced fecundity, including reduced numbers of eggs, and consequently decreased population size (Miller and Ankley, 2004; Miller et al., 2007). This AOP is potentially applicable to oviparous vertebrates in general because of the common function of VTG. Furthermore, many endocrine and other pathways, such as the function of aromatase in converting testosterone to E2, are conserved among fish and mammals, and therefore several fish AOPs can also be useful from the perspective of human toxicology. The AOP is part of the OECD AOP development program work plan and has gone through internal and external review. It was endorsed by the Working Group of the National Coordinators for the Test Guidelines Program and has been published by the OECD (Villeneuve, 2016).
Figure 5.
AOP from aromatase inhibition leading to reproductive dysfunction in fish (https://aopwiki.org/aops/25).
In contrast to the skin sensitization AOP where all KEs, except for the AO, can be measured using alternative assays, the aromatase inhibition AOP involves the function of feedback loops regulating hormone levels and linkages all the way up to reproductive capacity, which cannot all be represented using in vitro assays. To move away from the need to measure the KEs and AO in animal tests, computational models have been developed to take in vivo regulatory processes into account and predict effects along the AOP starting from the potency of a chemical to inhibit aromatase. These models have then been coupled together to form a quantitative AOP (Conolly et al., 2017, Wittwehr et al., 2017). The first component of this quantitative AOP is a hypothalamic-pituitary-gonadal (HPG) axis model (Figure 5) predicting VTG production based on aromatase inhibition potential (Cheng et al., 2016). The model includes two feedback loops, the first accounting for upregulation of aromatase activity in response to decreased E2 levels, and the second accounting for upregulation of a transporter for VTG uptake into the ovary in response to decreased ovarian VTG levels. The second component of the quantitative AOP is the oocyte growth dynamics model predicting oocyte development and spawning based on plasma VTG levels (Li et al., 2011). The third component is a population model predicting density-dependent population trajectories based on fecundity (Miller and Ankley, 2004). By coupling these three models together, it becomes possible to predict the probability or severity of the AO, whereas a qualitative AOP only describes the plausible sequence of events assuming that compensatory and repair mechanisms are overwhelmed. This quantitative AOP can be applied to estimate a benchmark dose for an untested chemical (Conolly et al. 2017). The potency of a chemical to inhibit aromatase relative to the model compound, which was used to develop the models, can serve as input for the predictions. A cell-free aromatase inhibition test is included in the ToxCast battery of assays and can provide the necessary input data (Richard et al., 2016). The modular structure of this quantitative AOP allows for the use of higher level KE data when available, such as plasma VTG levels, and similar to the concept of modularity of AOPs, it allows for the re-use of model components in other AOPs and eventually for the construction of quantitative AOP networks. This illustrates the role AOPs can play in 21st century predictive toxicology (NRC, 2007), by predicting apical AOs of regulatory concern based on mechanistic endpoints measured with alternative assays and thus reducing the need for animal tests.
6. Perspectives
Risk evaluation of chemicals based on an understanding of the responsible mechanisms has been a goal since generations of toxicologists, and although the biological concepts behind the AOP framework are in principle not new, AOPs have found their way to the fields of toxicology and risk assessment in recent years. Despite the introduction of guidance documents and papers on AOP development and assessment (Becker et al., 2015; OECD, 2013; Villeneuve et al., 2014a and 2014b), the AOP framework and its applications and will undoubtedly benefit from optimization and further development in the upcoming years. For example, a frequently raised criticism on individual AOPs is that they oversimplify the complexity of biological systems and the exposures to stressors that these systems face. Most realistic exposure scenarios involve multiple stressors (i.e. mixtures) and even when considering exposure to single chemicals, multiple AOPs may be triggered. Thus, in most scenarios, AOPs cannot be considered in isolation. It should, however, be kept in mind that individual AOPs are merely viewed as pragmatic units for development. Risk assessment applications will necessitate the use of AOP networks that combine individual AOPs (Knapen et al., 2015). AOP networks could potentially link many different MIEs to different AOs, describe specific combinations of KEs or pathways that are required for progression towards an AO, and could be used to more realistically describe mixture toxicity responses. In addition to being able to more accurately describe complex toxicological processes, identification of common KEs in such AOP networks will be valuable for developing assays that are indicative of different adverse effects of regulatory concern at once.
In general, AOPs are to be considered as an open and flexible knowledge management tool that should be continuously refined by feeding in relevant data (Villeneuve et al., 2014a). Such iterative refinement should ideally include the elaboration and quantification of the dynamic properties of KEs and KERs. Although quantification has now been pursued for a limited number of AOPs, the vast majority of them are still qualitative in nature. That said, it should be kept in mind that AOPs of qualitative nature, but with a strong evidence base, can prove extremely valuable for many regulatory applications. Striving for more explicit, quantitative AOPs will inevitably require more consideration of how to optimally capture important aspects of system dynamics in AOPs and AOP networks. Dynamic motifs, such as feedback and feedforward loops, modulating factors and compensatory mechanisms drive, for example, adaptive or acclimation responses, but also determine critical tipping points for progression of one KE to the next. Although such information can currently already be captured within KER and/or KE descriptions, having more scope to explicitly incorporate and structure dynamic aspects into AOPs would evolve the AOP framework to be capable of reflecting higher resolution of biological realism in cases or applications where there is a need or desire to do so. Finally, besides enhancing the AOP framework to better capture toxicodynamic processes, it is important to acknowledge that the risk assessment paradigm equally relies on consideration of exposure scenarios and the integration of exposure data. Although AOPs are not chemical-specific and by design do not incorporate kinetics, additional efforts are indeed needed to bridge the gap between classical kinetic determinants related to ADME profiles of chemicals on the one hand and the activation of the MIE of an AOP on the other hand. To enable full exploitation of AOPs for risk assessment purposes and hence to appropriately reflect in vivo toxicological processes, conceptual frameworks should be developed to use chemical-specific kinetic data as the input for the chemical-specific application of chemical-agnostic AOPs in risk assessment scenarios. One recent example in this respect is the aggregate exposure pathway (AEP) framework (Teeguarden et al., 2016). The AEP concept is to exposure sciences what AOPs are to toxicological sciences, and AEP development and description intentionally draws as many parallels to AOP development as possible. Like AOPs, AEPs consist of KEs that are linked through KERs and describe the movement of specific chemicals from a source, such as industrial release, to the target site exposure, including environmental fate and transport, external exposure, and ADME. The AEP’s target site exposure could be directly linked to an AOP’s MIE, and AEPs and AOPs have thus been conceived as natural and complementary companions completing the source to outcome continuum.
Given the worldwide interest and initiatives taken in the AOP field, it is anticipated that these challenges discussed above will be addressed in the near future. The AOP program relies on expert-driven input to build the AOP-KB and therefore requires stakeholder engagement spanning diverse fields. In this regard, a recent Horizon Scanning effort supported by the Society of Environmental Toxicology and Chemistry (SETAC) identified current key questions surrounding the AOP framework by surveying the broader scientific and regulatory communities (Lalone et al., 2017). These questions have been then addressed within the context of a SETAC Pellston workshop held in the spring of 2017, with discussions focusing on elaborating principles and best practices concerning quantitative AOPs and AOP networks, amongst others to better capture dynamic motifs and modulating factors, and also on issues influencing more widespread engagement, uptake and application of the AOP framework to achieve greater international impact in both scientific and regulatory communities. The output of the workshop will be published in a series of related papers which will likely form the foundation for further development of the AOP framework.
Acknowledgements
This work was supported by the grants of the European Research Council (ERC Starting Grant 335476), the Fund for Scientific Research-Flanders (FWO grants G009514N, G010214N and G051117N), the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-VUB) and the Cefic Long-range Research Initiative project LRI-ECO20.2 with support of ECETOC. The authors would like to thank Dr. Steve Edwards, Dr. Dan Villeneuve and Dr. John Vandenberg for their technical peer review.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- AEP
aggregate exposure pathway
- AO(s)
adverse outcome(s)
- AOP(s)
adverse outcome pathway(s)
- AOP-KB
adverse outcome pathway knowledge base
- ARE
anti-oxidant response element
- DPRA
direct peptide reactivity assay
- E2
17-beta-estradiol
- EPA
Environmental Protection Agency
- FELS
fish early life-stage
- GPMT
Guinea pig maximization test
- hCLAT
human cell line activation test
- HPG
hypothalamic-pituitary-gonadal
- IATA
integrated approaches to testing and assessment
- JRC
Joint Research Centre of the European Commission
- Keap1/Nrf2
Kelch-like ECH-associates protein1/nuclear erythroid 2-related factor
- KE(R)
key event (relationship)
- LLNA
local lymph node assay
- MIE
molecular initiating event
- MUSST
myeloid U937 skin sensitization test
- OECD
Organization for Economic Cooperation and Development
- QSAR
quantitative structure-activity relationship
- REACH
Registration, Evaluation, Authorization and Restriction of Chemicals
- SETAC
Society of Environmental Toxicology and Chemistry
- VTG
vitellogenin
Footnotes
Disclaimer
The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Ankley GT, Bencic DC, Cavallin JE, Jensen KM, Kahl MD, Makynen EA, Martinovic D, Mueller ND, Wehmas LC, Villeneuve DL. Dynamic nature of alterations in the endocrine system of fathead minnows exposed to the fungicide prochloraz. Toxicol Sci. 2009;112:344–353. doi: 10.1093/toxsci/kfp227. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Ashikaga T, Yoshida Y, Hirota M, Yoneyama K, Itagaki H, Sakaguchi H, Miyazawa M, Ito Y, Suzuki H, Toyoda H. Development of an in vitro skin sensitization test using human cell lines: the human cell line activation test (h-CLAT): I. optimization of the h-CLAT protocol. Toxicol In Vitro. 2006;20:767–773. doi: 10.1016/j.tiv.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Basketter DA, White IR, McFadden JP, Kimber I. Skin sensitization: implications for integration of clinical data into hazard identification and risk assessment. Hum Exp Toxicol. 2015;34:1222–1230. doi: 10.1177/0960327115601760. [DOI] [PubMed] [Google Scholar]
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, et al. Increasing scientific confidence in adverse outcome pathways: application of tailored Bradford-Hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 2015;72:514–537. doi: 10.1016/j.yrtph.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Bell SM, Angrish MM, Wood CE, Edwards SW. Integrating Publicly Available Data to Generate Computationally Predicted Adverse Outcome Pathways for Fatty Liver. Toxicol Sci. 2016;150:510–520. doi: 10.1093/toxsci/kfw017. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Cohen SM, Dellarco V, McGregor D, Meek ME, Vickers C, Willcocks D, Farland W. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 2006;36:781–92. doi: 10.1080/10408440600977677. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, Seed J, Vickers C. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol. 2008;38:87–96. doi: 10.1080/10408440701749421. [DOI] [PubMed] [Google Scholar]
- Burden N, Sewell F, Andersen ME, Boobis A, Chipman JK, Cronin MT, Hutchinson TH, Kimber I, Whelan M. Adverse outcome pathways can drive non-animal approaches for safety assessment. J Appl Toxicol. 2015;35:971–975. doi: 10.1002/jat.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WY, Zhang Q, Schroeder A, Villeneuve DL, Ankley GT, Conolly R. Computational Modeling of Plasma Vitellogenin Alterations in Response to Aromatase Inhibition in Fathead Minnows. Toxicol Sci. 2016;154:78–89. doi: 10.1093/toxsci/kfw142. [DOI] [PubMed] [Google Scholar]
- Collier ZA, Gust KA, Gonzalez-Morales B, Gong P, Wilbanks MS, Linkov I, Perkins EJ. A weight of evidence assessment approach for adverse outcome pathways. Regul Toxicol Pharmacol. 2016;75:46–57. doi: 10.1016/j.yrtph.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Ankley GT, Cheng W, Mayo ML, Miller DH, Perkins EJ, Villeneuve DL, Wantanbe KH. Quantitative Adverse Outcome Pathways and Their Application to Predictive Toxicology. Environ Sci Technol. 2017;51:4661–4672. doi: 10.1021/acs.est.6b06230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JL, Jay Zhao Q, Lambert JC, Hawkins BS, Thomas RS, Wesselkamper SC. Application of Gene Set Enrichment Analysis for Identification of Chemically-Induced, Biologically Relevant Transcriptomic Networks and Potential Utilization in Human Health Risk Assessment. Toxicol Sci. 2017 doi: 10.1093/toxsci/kfx021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellarco VL, Wiltse JA. US Environmental Protection Agency's revised guidelines for Carcinogen Risk Assessment: incorporating mode of action data. Mutat Res. 1998;405:273–277. doi: 10.1016/s0027-5107(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Delrue N, Sachana M, Sakuratani Y, Gourmelon A, Leinala E, Diderich R. The adverse outcome pathway concept: A basis for developing regulatory decision-making tools. Altern Lab Anim. 2016;44:417–429. doi: 10.1177/026119291604400504. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA. Adverse outcome pathways: organizing toxicological information to improve decision making. J Pharmacol Exp Ther. 2016;356:170–181. doi: 10.1124/jpet.115.228239. [DOI] [PubMed] [Google Scholar]
- Emter R, Ellis G, Natsch A. Performance of a novel keratinocyte-based reporter cell line to screen skin sensitizers in vitro. Toxicol Appl Pharmacol. 2010;245:281–290. doi: 10.1016/j.taap.2010.03.009. [DOI] [PubMed] [Google Scholar]
- EPA. 2017 https://www.epa.gov/chemical-research/adverse-outcome-pathway-aop-research-brief (consulted April 2017)
- Ezendam J, Braakhuis HM, Vandebriel RJ. State of the art in non-animal approaches for skin sensitization testing: from individual test methods towards testing strategies. Arch Toxicol. 2016;90:2861–2883. doi: 10.1007/s00204-016-1842-4. [DOI] [PubMed] [Google Scholar]
- Gerberick GF, Vassallo JD, Bailey RE, Chaney JG, Morrall SW, Lepoittevin JP. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 2004;81:332–343. doi: 10.1093/toxsci/kfh213. [DOI] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- Hinfray N, Porcher JM, Brion F. Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Compar Biochem Physiol C. 2006;144:252–262. doi: 10.1016/j.cbpc.2006.09.002. [DOI] [PubMed] [Google Scholar]
- http://aopkb.org/ (consulted April 2017)
- https://www.qsartoolbox.org/ (consulted April 2017)
- Kimber I, Mitchell JA, Griffin AC. Development of a murine local lymph node assay for the determination of sensitizing potential. Food Chem Toxicol. 1986;24:585–586. [Google Scholar]
- Knapen D, Vergauwen L, Villeneuve DL, Ankley GT. The potential of AOP networks for reproductive and developmental toxicity assay development. Repr Toxicol. 2015;56:52–55. doi: 10.1016/j.reprotox.2015.04.003. [DOI] [PubMed] [Google Scholar]
- LaLone CA, Ankley GT, Belanger SE, Embry MR, Hodges G, Knapen D, Munn S, Perkins EJ, Rudd MA, Villeneuve DL, Whelan M, et al. Advancing the adverse outcome pathway framework: an international horizon scanning approach. Environ Toxicol Chem. 2017 doi: 10.1002/etc.3805. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Villeneuve DL, Jensen KM, Ankley GT, Watanabe KH. A computational model for asynchronous oocyte growth dynamics in a batch-spawning fish. Can J Fisheries Aquat Sci. 2011;68:1528–1538. [Google Scholar]
- Magnusson B, Kligman AM. The identification of contact allergens by animal assay: the Guinea pig maximization test. J Invest Dermatol. 1969;52:268–276. doi: 10.1038/jid.1969.42. [DOI] [PubMed] [Google Scholar]
- Maxwell G, MacKay C, Cubberley R, Davies M, Gellatly N, Glavin S, Gouin T, Jacquoilleot S, Moore C, Pendlington R, Saib O, et al. Applying the skin sensitisation adverse outcome pathway (AOP) to quantitative risk assessment. Toxicol In Vitro. 2014;28:8–12. doi: 10.1016/j.tiv.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Meek ME, Boobis A, Cote I, Dellarco V, Fotakis G, Munn S, Seed J, Vickers C. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J Appl Toxicol. 2014a;34:1–18. doi: 10.1002/jat.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek ME, Palermo CM, Bachman AN, North CM, Jeffrey Lewis R. Mode of action human relevance (species concordance) framework: evolution of the Bradford-Hill considerations and comparative analysis of weight of evidence. J Appl Toxicol. 2014b;34:595–606. doi: 10.1002/jat.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DH, Ankley GT. Modeling impacts on populations: fathead minnow (Pimephales promelas) exposure to the endocrine disruptor 17 beta-trenbolone as a case study. Ecotoxicol Environ Safety. 2004;59:1–9. doi: 10.1016/j.ecoenv.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Miller DH, Jensen KM, Villeneuve DL, Kahl MD, Makynen EA, Durhan EJ, Ankley GT. Linkage of biochemical responses to population-level effects: a case study with vitellogenin in the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2007;26:521–527. doi: 10.1897/06-318r.1. [DOI] [PubMed] [Google Scholar]
- Nelson K, Schroeder A, Ankley G, Blackwell B, Blanksma C, Degitz S, Flynn K, Jensen K, Johnson R, Kahl M, Knapen D, et al. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part I: Fathead minnow. Aquat Toxicol. 2016;173:192–203. doi: 10.1016/j.aquatox.2015.12.024. [DOI] [PubMed] [Google Scholar]
- NRC. Toxicity testing in the 21st Century: a vision and a strategy. The National Academies Press; Washington DC, USA: 2007. [Google Scholar]
- OECD. OECD series on testing and assessment number 168: the adverse outcome pathway for skin sensitization initiated by covalent binding to proteins part 1: scientific evidence ENV/JM/MONO(2012)10/PART1. OECD Publishing; Paris: 2012. [Google Scholar]
- OECD. OECD series on testing and assessment number 184: guidance document on developing and assessing adverse outcome pathways ENV/JM/MONO(2013)6. OECD Publishing; Paris: 2013. [Google Scholar]
- OECD. The adverse outcome pathway for skin sensitisation initiated by covalent binding to proteins. OECD Publishing; Paris: 2014. [Google Scholar]
- OECD. OECD series on adverse outcome pathways number 1: users' handbook supplement to the guidance document for developing and assessing adverse outcome pathways. OECD Publishing; Paris: 2016. [Google Scholar]
- Oki NO, Edwards SW. An integrative data mining approach to identifying adverse outcome pathway signatures. Toxicology. 2016;350–352:49–61. doi: 10.1016/j.tox.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Oki NO, Nelms MD, Bell SM, Mortensen HM, Edwards SW. Accelerating Adverse Outcome Pathway Development Using Publicly Available Data Sources. Curr Environ Health Rep. 2016;3:53–63. doi: 10.1007/s40572-016-0079-y. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Kuseva C, Kesova A, Popova I, Zhechev T, Pavlov T, Roberts DW, Mekenyan O. Towards AOP application: implementation of an integrated approach to testing and assessment (IATA) into a pipeline tool for skin sensitization. Regul Toxicol Pharmacol. 2014;69:529–545. doi: 10.1016/j.yrtph.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Simon TW, Rowlands JC, Budinsky RA, Becker RA. Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Regul Toxicol Pharmacol. 2015;71:463–477. doi: 10.1016/j.yrtph.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Macherla C, Filer DL, Burgess E, Simmons SO, Crofton KM, Hornung MW. Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology. 2013;312:97–107. doi: 10.1016/j.tox.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Rotroff DM, Hornung MW, Crofton KM, Simmons SO. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem Res Toxicol. 2014;27:387–99. doi: 10.1021/tx400310w. [DOI] [PubMed] [Google Scholar]
- Perkins EJ, Antczak P, Burgoon L, Falciani F, Garcia-Reyero N, Gutsell S, Hodges G, Kienzler A, Knapen D, McBride M, Willett C. Adverse outcome pathways for regulatory applications: examination of four case studies with different degrees of completeness and scientific confidence. Toxicol Sci. 2015;148:14–25. doi: 10.1093/toxsci/kfv181. [DOI] [PubMed] [Google Scholar]
- Preston RJ, Williams GM. DNA-reactive carcinogens: mode of action and human cancer hazard. Crit Rev Toxicol. 2005;35:673–83. doi: 10.1080/10408440591007278. [DOI] [PubMed] [Google Scholar]
- Python F, Goebel C, Aeby P. Assessment of the U937 cell line for the detection of contact allergens. Toxicol Appl Pharmacol. 2007;220:113–124. doi: 10.1016/j.taap.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Ramirez T, Mehling A, Kolle SN, Wruck CJ, Teubner W, Eltze T, Aumann A, Urbisch D, van Ravenzwaay B, Landsiedel R. LuSens: a keratinocyte based ARE reporter gene assay for use in integrated testing strategies for skin sensitization hazard identification. Toxicol In Vitro. 2014;28:1482–1497. doi: 10.1016/j.tiv.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Ashikaga T, Miyazawa M, Yoshida Y, Ito Y, Yoneyama K, Hirota M, Itagaki H, Toyoda H, Suzuki H. Development of an in vitro skin sensitization test using human cell lines: human cell line activation test (h-CLAT): II. An interlaboratory study of the h-CLAT. Toxicol In Vitro. 2006;20:774–784. doi: 10.1016/j.tiv.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, et al. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol. 2001;34:146–152. doi: 10.1006/rtph.2001.1493. [DOI] [PubMed] [Google Scholar]
- Stinckens E, Vergauwen L, Schroeder AL, Maho W, Blackwell BR, Witters H, Blust R, Ankley GT, Covaci A, Villeneuve DL, Knapen D. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: zebrafish. Aquat Toxicol. 2016;173:204–217. doi: 10.1016/j.aquatox.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Teeguarden JG, Tan YM, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanguay RL, Waters KM, et al. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ Sci Technol. 2016;50:4579–4586. doi: 10.1021/acs.est.5b05311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, Garcia-Reyero N, Hartung T, Worth A, Patlewicz G. Applying adverse outcome pathways (AOPs) to support integrated approaches to testing and assessment (IATA) Regul Toxicol Pharmacol. 2014;70:629–640. doi: 10.1016/j.yrtph.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Urbisch D, Mehling A, Guth K, Ramirez T, Honarvar N, Kolle S, Landsiedel R, Jaworska J, Kern PS, Gerberick F, Natsch A, et al. Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul Toxicol Pharmacol. 2015;71:337–351. doi: 10.1016/j.yrtph.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Mueller ND, Martinovic D, Makynen EA, Kahl MD, Jensen KM, Durhan EJ, Cavallin JE, Bencic D, Ankley GT. Ankley GT Direct effects, compensation, and recovery in female fathead minnows exposed to a model aromatase inhibitor. Environ Health Persp. 2009;117:624–631. doi: 10.1289/ehp.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, et al. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci. 2014a;142:312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. Adverse outcome pathway development II: best practices. Toxicol Sci. 2014b;142:321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Volz DC, Embry MR, Ankley GT, Belanger SE, Leonard M, Schirmer K, Tanguay R, Truong L, Wehmas L. Investigating alternatives to the fish early-life stage test: a strategy for discovering and annotating adverse outcome pathways for early fish development. Environ Toxicol Chem. 2014c;33:158–169. doi: 10.1002/etc.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL. OECD Series on Adverse Outcome Pathways number 4. OECD Publishing; Paris: 2016. Adverse outcome pathway on aromatase inhibition leading to reproductive dysfunction (in fish) [Google Scholar]
- Wang CC, Lin YC, Wang SS, Shih C, Lin YH, Tung CW. SkinSensDB: a curated database for skin sensitization assays. J Cheminform. 2017;9:5. doi: 10.1186/s13321-017-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltse JA, Dellarco VL. U.S. Environmental Protection Agency's revised guidelines for carcinogen risk assessment: evaluating a postulated mode of carcinogenic action in guiding dose-response extrapolation. Mutat Res. 2000;464:105–115. doi: 10.1016/s1383-5718(99)00171-0. [DOI] [PubMed] [Google Scholar]
- Wittwehr C, Aladjov H, Ankley G, Byrne HJ, de Knecht J, Heinzle E, Klambauer G, Landesmann B, Luijten M, MacKay C, Maxwell G, et al. How adverse outcome pathways can aid the development and use of computational prediction models for regulatory toxicology. Toxicol Sci. 2017;155:326–336. doi: 10.1093/toxsci/kfw207. [DOI] [PMC free article] [PubMed] [Google Scholar]