Abstract
Objective
To evaluate the relationship between pro-atherogenic biomarkers and epicardial adipose tissue (EAT) thickness in patients with cardiovascular risk factors.
Methods
Plasma nitric oxide (NO), soluble intercellular adhesion molecule-1 and malondialdehyde (MDA) levels, EAT thickness, flow-mediated dilation (FMD) and carotid intima media thickness (CIMT) were determined in patients aged >18 years who were referred for echocardiography for heart ischemia or non-ischemic diseases. Cardiovascular risk factors (Framingham score [FS] ≥ 20) were weighted.
Results
Hypertension, dyslipidaemia and type 2 diabetes mellitus were prevalent (≥55% of 40 patients). Patients with FS ≥ 20 (n = 21) showed significantly higher EAT and CIMT values. Globally, MDA, CIMT, age, waist circumference, high-density lipoprotein cholesterol (HDL-C) and FS were associated with EAT thickness. EAT was significantly associated with NO in patients with FS ≥ 20. Significant differences in EAT thickness were found between patients stratified by NO value, FMD, age, smoking status, dyslipidaemia, type 2 diabetes mellitus and FS. An EAT-associated atherogenic risk (CIMT ≥ 1 mm) model was statistically significant when MDA and type 2 diabetes mellitus were included.
Conclusion
EAT thickness was associated with MDA, CIMT, age, waist circumference, HDL-C and FS globally, but with NO only in patients with FS≥20. EAT may be used to identify vascular damage stage, possibly influenced by MDA and type 2 diabetes mellitus.
Keywords: Epicardial adipose tissue, nitric oxide, malondialdehyde, atherogenesis, cardiovascular risk
Introduction
Epicardial adipose tissue (EAT), a type of visceral adipose tissue, produces molecules that have angiocrine, vasomotor and pro-inflammatory effects and regulate blood flow to the coronary arteries.1 The generation of reactive oxygen species parallels the production of pro-inflammatory mediators, and both exert synergistic damage on the endothelium in patients with high cardiovascular risk.2
Pathophysiological evidence supports a role for EAT in atherogenic processes. Experimentally, EAT thickness is negatively correlated with local adiponectin expression and positively correlated with leptin, resistin and TNF-α.3 This impairs endothelial function by affecting endothelial nitric oxide (NO) synthase-dependent pathways and decreases endothelial protective effects as a result of a lower modulatory effect on oxidative stress.3 Moreover, EAT thickness has been associated with the expression of pro-atherogenic biomarkers, including NO, soluble intercellular adhesion molecule-1 (sICAM-1) and biomarkers of oxidative stress and the endocannabinoid system in patients with coronary artery disease or subclinical cardiovascular risk.4–6 Clinically, the relationship between EAT and endothelial dysfunction and atherogenesis has been evaluated using assessments such as flow-mediated dilation (FMD) tests and carotid intima media thickness (CIMT), which are commonly used as non-invasive markers of endothelial function.7,8 EAT thickness is an accessible, non-invasive measurement that can be obtained using a routine bi-dimensional echocardiographic scan, and can be identified as the space between the external layer of the myocardium and the visceral layer of the pericardium.9 Since studies have indicated a relationship between EAT thickness and early mediators of endothelial damage and atherogenesis, EAT thickness has become a clinically relevant measure to estimate atherogenic risk.1
Specific mechanisms that are linked to conditions such as obesity are suggested to influence the relationship between EAT thickness and coronary artery disease.10,11 Such findings suggest that measuring EAT thickness with biomarkers of endothelial damage may be clinically useful for achieving a more accurate stratification of a patient's atherogenic risk. Thus, the aim of the present study was to evaluate whether endothelial damage mediators participate in the relationship between EAT thickness and subclinical atherogenesis in a study population that was stratified by conventional cardiovascular risk factors.
Patients and methods
Study population
This prospective cross-sectional cohort study included patients >18 years of age who were evaluated in the Department of Echocardiography, National Medical Center ‘20 de Noviembre’ ISSSTE, Mexico City, Mexico between November 2015 and March 2016 following referral by health-care units that serve the general population in Mexico City. Echocardiography was performed as a follow-up procedure in patients with coronary artery disease or during a routine evaluation in non-ischemic patients who displayed a variable number of cardiovascular risk factors, and in whom heart disease was suspected. Patients undergoing echocardiography were sequentially enrolled according to a Framingham score (FS)12 of ≥ 20 or < 20, to obtain balanced groups stratified according to a cut-off for significant conventional cardiovascular risk factors (considered to be significant if FS ≥20%).
Patients with pericardial effusion or peripheral artery disease were excluded, as were patients who had undergone heart surgery, such as coronary artery bypass grafting or aortic and mitral valve repair or replacement, due to the possibility that EAT had been manipulated during the surgical procedure. Patients with stroke were also excluded to focus the analyses on conventional cardiovascular risk factors without undue influence by other factors, such as cardio embolism.
Smoking history was obtained from patients during a medical interview, and nonsmokers were defined as having never smoked during their lifetime. The number of pack years was calculated as the number of cigarettes smoked per day/20, multiplied by the time (years) smoked.
This study was designed and performed according to ethical guidelines of the 1975 Declaration of Helsinki, and approved by the Local Committees of Research, Ethics in Research and Biosafety of the Centro Médico Nacional ‘20 de Noviembre’ ISSSTE, Mexico City (Protocol ID No. 386.2013). All participants provided written informed consent.
Anthropometry and blood pressure
Body mass index was calculated as weight/height2 (kg/m2). Waist circumference was measured half way between the lowest rib margin and the iliac crest at the end of a normal expiration. Hip circumference was measured around the widest portion of the buttocks with the tape parallel to the floor. The waist-to-hip ratio was calculated by dividing waist circumference by hip circumference. Blood pressure was obtained while the patient was in a seated position and was considered to be the mean of three readings taken 5 min apart using a Welch Allyn 767 mobile aneroid sphygmomanometer (Welch Allyn Inc.; Skaneateles Falls, NY, USA).
Measurement of plasma biomarkers
Following a 12-h fast, venous blood samples (4 ml) were collected into BD Vacutainer® tubes (Becton, Dickinson & Co., Franklin Lakes, NJ, USA) containing 1.8 mg ethylenediaminetetra-acetic acid/ml of blood, centrifuged at 1 200 g for 10 min at 4 ℃, separated into fractions and stored at −80 ℃ prior to analyses. Plasma levels of triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glycosylated haemoglobin (HbA1c) and glucose were determined using routine clinical laboratory equipment and a standard auto-analyser (Synchron CX®9 PRO Clinical System; Beckman Coulter, Brea, CA, USA).
Plasma markers were analysed in the Research Laboratory of Experimental Metabolism and Clinical Research, Centro Médico Nacional ‘20 de Noviembre’ ISSSTE, Mexico City: sICAM-1 levels were determined using commercially available immunoassay kits (Quantikine® enzyme-linked immunosorbent assay: Human ICAM-1/CD54 Allele-specific Immunoassay; R&D Systems Inc., Minneapolis, MN, USA). Malondialdehyde (MDA) levels were determined using a published method based on the reaction of MDA with thiobarbituric acid at 95 ℃,13 and absorbance of the resultant pink colour was read at a wavelength of 532 nm. Between the first and second month following sample collection, NO levels were determined by the cadmium-mediated reduction of nitrates to nitrites, and subsequent measurement of nitrite concentration using a colorimetric Griess assay.14
Type 2 diabetes mellitus was defined using guidelines of the American Diabetes Association15 in patients having one of the following conditions (repeated for confirmation at a separate date [JAP-Z]): (1) HbA1c ≥ 6.5%; (2) fasting glucose ≥126 mg/dl; or (3) 2-h plasma glucose ≥200 mg/dl during an oral glucose tolerance test. Hypertension was defined as blood pressure ≥ 140/90 mmHg. Dyslipidaemia was defined as one or more of the following conditions: (1) total cholesterol ≥ 200 mg/dl; (2) LDL-C ≥ 100 mg/dl; (3) HDL-C< 40 mg/dl, or (4) triglycerides ≥ 150 mg/dl.
Diagnostic imaging
To determine EAT thickness, two-dimensional, M-mode and Doppler transthoracic echocardiography were performed in the left lateral position using a Philips iE33 cardiac ultrasound system (Philips Medical Systems, Andover, MA, USA) and a Phillips iE33 S5-1 Transducer (Royal Philips, Amsterdam, The Netherlands). EAT thickness was defined as the space between the visceral layer of the pericardium and the outer border of the myocardium, which registered on the standard parasternal longitudinal axis and transverse images. The aortic ring was used as the anatomical reference for the large axis parasternal view, and the papillary muscles were used for the short axis. The images were acquired in bi-dimensional mode, perpendicular to the free wall of the right ventricle, at the end of systole, over three cardiac cycles.
In addition, the following echocardiographic parameters were measured using the guidelines of the American Society of Echocardiography: left ventricle ejection fraction was measured using a modified Simpson method.16 The global longitudinal strain (fractional change, expressed as a positive or negative percentage, that reflects the lengthening or shortening of a myocardial segment, respectively) was measured as the mean of the left ventricular segments longitudinal strains.17 Diastolic dysfunction (the decline in ventricular performance during diastole, due to abnormal myocardial relaxation and passive left ventricular pressures) was classified as follows: Grade I (mild), if mitral E/A ratio < 0.8 (where E = early diastole wave and A = end-diastole [atrial contraction] wave), deceleration time > 200 ms, isovolumetric relaxation time ≥100 ms, predominant systolic flow is observed in pulmonary venous inflow (S/D > 1 [where S = peak systolic flow and D = peak anterograde diastolic flow]), e' < 8 cm/s (where e' = diastolic peak velocity of the movement of the annulus towards the left atrium during initial filling of the left ventricle) and E/e' ratio < 8, either septal or lateral; Grade II (moderate), if mitral E/A ratio = 0.8–1.5 (pseudonormal) and decreases by 50% during the Valsalva manoeuver, e' < 8 cm/s and E/e' ratio = 9–12 (additional supporting data include a PV-Ar velocity > 30 cm/s [where PV-Ar = end-diastole pulmonary venous flow] and S/D ratio<1); or Grade III (severe), if restrictive left ventricular filling occurs accompanied by an E/A ratio ≥ 2, deceleration time < 160 ms, isovolumetric relaxation time ≤60 ms, systolic filling fraction ≤40%, mitral A flow duration shorter than PV-Ar duration and E/e' ratio > 13 (or septal E/e' ratio ≥ 15 and lateral E/e' ratio > 12).18
An FMD test was used to measure the diameter of the brachial artery above the antecubital fossa, which was evaluated using ultrasound imaging with a Philips EPIQ7 L12-3 Broadband Linear Array Transducer (Royal Philips) in B-mode according to the recommendations of the International Brachial Artery Reactivity Task Force.19 The same equipment was used to measure CIMT.20 Briefly, with the patient in a supine position, common carotid artery scanning was initiated while focusing on the posterior carotid wall at the beginning of the carotid bifurcation and the common carotid artery. At least four measurements were obtained at a distance of approximately 10 mm proximal to where the carotid dilatation was performed because it represents the greatest distance between the lumen-intima interface and the media adventitia interface. In all of the cases, reproducibility of the measurement was validated using acceptable intraclass correlation coefficients (agreement) for inter-observer reliability.
Statistical analyses
The study was designed to evaluate correlations between continuous response variables (pro-atherogenic biomarkers) and EAT in patients who were divided into balanced categorical groups, stratified according to cardiovascular risk factors (Framingham score ≥ 20 and presence of significant ischemic heart disease versus Framingham score <20 and no ischemic heart disease). To calculate the sample size, previously reported correlations between biomarkers of interest and EAT thickness were taken into account, then, observed significant differences in biomarker values for similar populations (grouped according to the presence of coronary artery disease) were considered. To reject the null hypothesis that the correlation between EAT and each pro-atherogenic biomarker is less significant than previously reported findings or that there is no correlation with a probability (power) of 0.80 and a type I (α) error probability of 0.05, the study was determined to require 40 patients, divided into balanced groups according to the presence or absence of significant ischemic heart disease.
To weight the effects of pro-atherogenic factors on EAT thickness, cut-off values were calculated for the following pro-atherogenic biomarkers using a receiver operating characteristic curve, which best discriminated CIMT ≥ 1: EAT; NO; sICAM-1 and MDA. A cut-off value for FMD was set at 4.5% in accordance with previous reports.21,22 EAT thickness values were then analysed in patients stratified according to biomarker cut-offs. EAT thickness values were similarly analysed for patients stratified according to clinical/demographic variables (age, smoking, dyslipidaemia, type 2 diabetes mellitus, Framingham score).
For descriptive statistics, continuous variables are presented as median (interquartile range), and categorical variables are presented as n (%) prevalence. Data distributions for the variables were estimated using Kolmogorov–Smirnov test. Between-group comparisons of continuous variables were performed using unpaired Student's t-tests or Mann–Whitney U-tests (2-tailed). Categorical variables were compared between groups using χ2-test or Fisher's exact test (as appropriate). Pearson's correlation coefficient was applied to determine the relationship between biomarkers and EAT values. Any biomarkers found to have a potential association with EAT and to be the best predictors of subclinical atherogenesis (CIMT > 1 mm) were identified using modelling in the ‘enter’ method during logistic regression analysis. The relative risk and 95% confidence intervals (CIs) are shown. All statistical analyses were performed using SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA) for Windows®, and P values ≤ 0.05 (2-tailed) were considered to be statistically significant.
Results
A total of 40 patients with a mean age of 59 years (range, 33–86 years) were included in the study (Table 1). Hypertension and dyslipidaemia, followed by type 2 diabetes mellitus, were the most frequent cardiovascular risk factors in the study population.
Table 1.
Demographic, clinical and echocardiographic characteristics of patients >18 years of age with coronary artery disease, or non-ischemic patients who displayed a variable number of cardiovascular risk factors, and in whom heart disease was suspected (n = 40).
| Characteristic | All patients | Framingham score |
|
|---|---|---|---|
| (n = 40) | <20 (n = 19) | ≥20 (n = 21) | |
| Age, years | 62.0 (49.0–69.0) | 46.0 (41.2–62.7) | 66.0 (61.5–72.5)a |
| Sex, male | 23 (57.5) | 8 (42.1) | 15 (71.4) |
| Smoker | 18 (45.0) | 4 (21.1) | 14 (66.6)b |
| Body mass index ≥ 30 | 15 (37.5) | 9 (47.4) | 6 (28.6) |
| WC (≥102cm [m], ≥ 88cm [f]) | 21 (52.5) | 8 (42.1) | 13 (61.9) |
| WtHR (≥1cm [m], ≥ 0.85cm [f]) | 24 (60.0) | 11 (57.9) | 13 (61.9) |
| High blood pressure, ≥ 140/90 mmHg | 25 (62.5) | 9 (47.4) | 16 (76.2) |
| Dyslipidaemia | 24 (60.0) | 6 (31.6) | 18 (85.7)b |
| Type 2 diabetes mellitus | 22 (55.0) | 15 (78.9) | 7 (33.3)b |
| Ischemic heart disease | 19 (47.5) | 0 (0.0) | 19 (90.5)b |
| Framingham score | 20.0 (4.5, 20.0) | 2.3 (1.0, 12.5) | 20.0 (20.0, 28.5)a |
| Echocardiographic data | |||
| EAT thickness, mm | 6.5 (4.8, 9.0) | 4.7 (3.9, 5.9) | 8.0 (6.4, 9.2)a |
| LVEF, % | 58.0 (49.0, 63.0) | 60.5 (55.0, 68.5) | 54.0 (46.5, 60.5)a |
| Global longitudinal strain, % | −17.9 (−13.5, −20.0) | −19.0 (−17.0, −21.0) | −15.0 (−12.0, −20.0)a |
| Diastolic dysfunction18 | |||
| none | 4 (10.0) | 4 (21.0) | 0 (0.0)b |
| mild | 29 (72.5) | 14 (73.7) | 15 (71.4) |
| moderate | 5 (12.5) | 1 (5.3) | 4 (19.0) |
| severe | 2 (5.0) | 0 (0.0) | 2 (9.6) |
Continuous variables presented as median (interquartile range); categorical data presented as n (%) patient prevalence.
Statistically significant differences (P < 0.05) between groups with a Framingham score <20 versus ≥ 20 following: a2-tailed Mann–Whitney U-test or Student's t-test; or bχ2-test or Fisher's exact test.
WC, waist circumference; WtHR, waist-to-hip ratio; [m], male; [f], female; EAT, epicardial adipose tissue; LVEF, left ventricle ejection fraction.
Cardiovascular risk factors may be differentially distributed between patients with and without coronary artery disease, therefore, the present study population were stratified based on the presence of ischemic heart disease and a Framingham score ≥20. The subgroup of patients with a Framingham score ≥20 were significantly older, had more smokers, and a higher prevalence of dyslipidaemia and ischemic heart disease, in addition to more affected heart function according to echocardiographic parameters (left ventricular ejection fraction, global longitudinal strain and absence of diastolic dysfunction; all P < 0.05; Table 1).
Values for EAT thickness were determined using echocardiography, and ranged from 3 to 13 mm for the whole study population. The left ventricle ejection fraction and strain values were within normal ranges (Table 1; where normal range for systolic left ventricular ejection fraction = 52–72% [male] and 54–74% [female], and global longitudinal strain = −16 to −22% [a peak of −20% is expected for a healthy person]).23,24 EAT thickness was significantly higher in patients with a Framingham score ≥20 (range, 5.2–11 mm) than in those with a Framingham score <20 (range, 3–8 mm; P < 0.05).
In terms of biomarkers that reflect subclinical atherogenesis (Table 2), patients with Framingham scores ≥20 had significantly lower FMD and higher CIMT values than the group with Framingham scores <20 (P < 0.05). No statistically significant between-group differences were observed in plasma biomarkers of endothelial dysfunction or oxidative stress (NO, sICAM-1 or MDA; Table 2).
Table 2.
Biomarkers of endothelial dysfunction and atherogenesis in patients >18 years of age with coronary artery disease, or non-ischemic patients who displayed a variable number of cardiovascular risk factors, and in whom heart disease was suspected.
| Atherogenic biomarker | All patients | Framingham score |
|
|---|---|---|---|
| (n = 40) | <20 (n = 19) | ≥20 (n = 21) | |
| NO, µmol/l | 34.4 (31.0–40.7) | 35.0 (33.0–39.8) | 32.6 (25.8–40.9) |
| sICAM-1, ng/ml | 112.2 (74.5–131.3) | 109.8 (75.3–139.6) | 114.2 (56.7–125.3) |
| MDA, nmol/ml | 0.99 (0.94–1.06) | 0.99 (0.89–1.12) | 0.98 (0.94–1.05) |
| FMD, % | 15.2 (4.7–35.3) | 35.6 (25.1–42.9) | 5.0 (2.8–14.0)a |
| CIMT, mm | 0.9 (0.7–1.1) | 0.7 (0.6–0.8) | 1.1 (0.9–1.2)a |
Continuous variables presented as median (interquartile range).
aStatistically significant differences (P < 0.05) between groups with a Framingham score <20 versus ≥ 20 (2-tailed Mann–Whitney U-test or Student's t-test).
NO, nitric oxide; sICAM-1, soluble intercellular adhesion molecule-1; MDA, malondialdehyde; FMD, flow-mediated dilation; CIMT, carotid intima-media thickness.
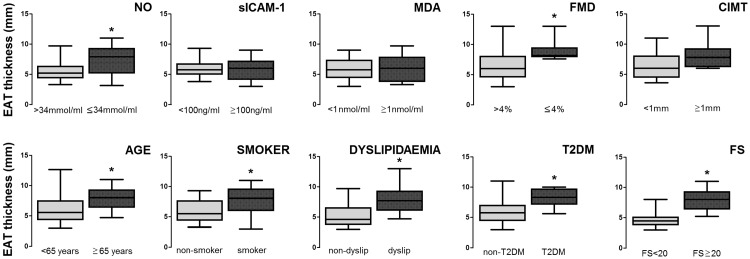
The weighted effects of pro-atherogenic factors on EAT thickness were analysed using the following biomarker cut-off values (calculated using a receiver operating characteristic curve, which best discriminated CIMT ≥ 1): EAT, 7.5 mm; NO, 34 µmol/ml; sICAM-1, 100 ng/ml and MDA, 1 nmol/ml. An FMD cut-off value of 4.5% was used, as previously stated. Patient groups were stratified by atherogenic biomarker (NO, sICAM-1, MDA, FMD, CIMT) or clinical/demographic variable (age, smoking, dyslipidaemia, type 2 diabetes mellitus, Framingham score), and EAT thickness was analysed (Figure 1). Statistically significant between-group differences in EAT thickness values were found in patient groups stratified by NO value, age, smoking status, dyslipidaemia, type 2 diabetes mellitus and Framingham score (P < 0.05; Figure 1).
Figure 1.
Association between pro-atherogenic factors and epicardial adipose tissue (EAT) thickness in patients > 18 years of age with coronary artery disease, or non-ischemic patients who displayed a variable number of cardiovascular risk factors, and in whom heart disease was suspected (n = 40). EAT thickness mean values are presented in patients: stratified by pro-atherogenic biomarkers (nitric oxide [NO], soluble intercellular adhesion molecule-1 [sICAM-1], malondialdehyde [MDA], flow-mediated dilation [FMD], and carotid intima media thickness [CIMT]) at calculated cut-off values to best discriminate CIMT≥1 (according to receiver operating characteristic curve analysis); and stratified by presence or absence of clinical-demographic variables associated with cardiovascular risk (age, smoking status, dyslipidaemia, type2 diabetes mellitus [T2DM], and Framingham score [FS]). *Statistically significant between-group difference (P < 0.05; Student's t-test or Mann–Whitney U-test [2-tailed] as appropriate). Box-whisker plots showing median (black horizontal line) with 25th and 75th percentiles (box extremities) and minimum and maximum values (error bars).
Correlation analyses were also performed to analyse the relationship between EAT thickness and NO, sICAM-1, MDA, FMD and CIMT in the whole patient cohort and in patients stratified by Framingham score (Table 3; due to the statistically significant differences in FMD, CIMT and EAT thickness observed between patients with Framingham scores ≥ 20 versus < 20). EAT thickness was significantly correlated with MDA (ρ = −0.54, P < 0.01) in the whole study cohort, but was not found to be significantly correlated with MDA when patients were stratified according to Framingham scores (Table 3). CIMT was found to be significantly correlated with EAT thickness in the whole study cohort (ρ = 0.51, P < 0.01) and in patient groups that were stratified according to Framingham scores (ρ = 0.46, P < 0.05 and ρ = 0.58, P < 0.02, Framingham score ≥ 20 and Framingham score < 20, respectively; Table 3). A statistically significant inverse correlation was found between EAT thickness and serum NO levels in the subgroup of patients with Framingham scores ≥ 20 (ρ = −0.56, P = 0.04), but not in the subgroup of patients with scores <20 (ρ = −0.15, P < 0.31; Table 3).
Table 3.
Association between atherogenic biomarkers and epicardial adipose tissue thickness in patients >18 years of age with coronary artery disease, or non-ischemic patients who displayed a variable number of cardiovascular risk factors, and in whom heart disease was suspected.
| Pearson's correlation coefficient (ρ) |
Statistical significance | ||
|---|---|---|---|
| Atherogenic biomarker | Mean | 95% CI | |
| All patients (n = 40) | |||
| NO | −0.26 | −0.61, 0.17 | NS |
| sICAM-1 | −0.13 | −0.52, 0.30 | NS |
| MDA | −0.54 | −0.78, 0.15 | P < 0.01 |
| FMD | −0.30 | 0.11, 0.76 | NS |
| CIMT | 0.51 | −0.64, 0.13 | P < 0.01 |
| Framingham score < 20 (n = 19) | |||
| NO | −0.15 | −0.66, 0.46 | NS |
| sICAM-1 | −0.07 | −0.62, 0.52 | NS |
| MDA | −0.50 | −0.83, 0.09 | NS |
| FMD | −0.09 | −0.63, 0.50 | NS |
| CIMT | 0.58 | 0.02, 0.86 | P = 0.02 |
| Framingham score ≥ 20 (n = 21) | |||
| NO | −0.56 | −0.88, 0.09 | P = 0.04 |
| sICAM-1 | −0.08 | −0.67, 0.57 | NS |
| MDA | −0.47 | −0.85, 0.22 | NS |
| FMD | −0.14 | −0.71, 0.53 | NS |
| CIMT | 0.46 | −0.23, 0.84 | P = 0.05 |
CI, confidence interval; NO, nitric oxide; sICAM-1, soluble intercellular adhesion molecule-1; MDA, malondialdehyde; FMD, flow-mediated dilation; CIMT, carotid intima-media thickness.
NS, no statistically significant correlation (P > 0.05; Pearson's correlation coefficient).
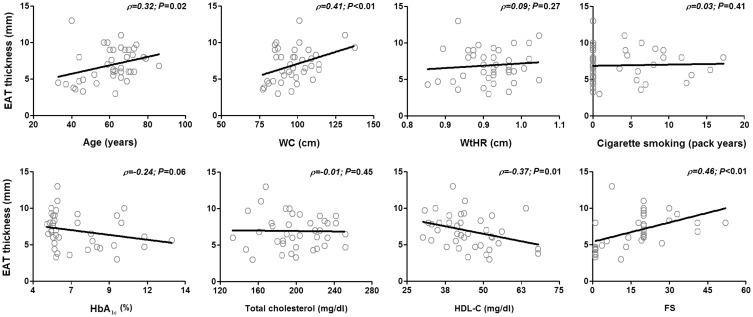
Further correlation analyses were performed to assess the association between EAT values and clinical and metabolically-relevant variables in the whole patient cohort. Age (ρ = 0.32, P = 0.02), waist circumference (ρ = 0.41, P = 0.01), HDL-C (ρ = −0.37, P = 0.01) and Framingham score (ρ = 0.46, P < 0.01) were found to be significantly associated with EAT thickness (Figure 2).
Figure 2.
Correlation between epicardial adipose tissue (EAT) thickness and relevant clinical/metabolic variables. Smoking pack years was calculated as: (number of cigarettes smoked per day/20) × time (years) smoked. EAT thickness was found to be significantly correlated with age, waist circumference (WC), high-density lipoprotein cholesterol (HDL-C) and Framingham score (FS). (P < 0.05; Pearson's correlation coefficient). WtHR, waist-to-hip ratio; HbA1c, glycosylated haemoglobin.
To determine whether biomarkers of endothelial dysfunction and metabolic damage affect the relationship between EAT and subclinical atherogenesis, logistic regression analyses were performed using the cut-off values for atherogenic biomarkers that were found to best discriminate CIMT ≥ 1, as described above. Different prediction models were generated (Table 4), and the association between EAT thickness and atherogenic progression (CIMT ≥ 1 mm) was only found to be statistically significant after type 2 diabetes mellitus and MDA were included in the model (Model 4; Table 4).
Table 4.
Logistic regression analysis using different prediction models of the association between subclinical atherogenesis (carotid intima-media thickness ≥ 1 mm) and epicardial adipose tissue (EAT) thickness in patients > 18 years of age with coronary artery disease, or non-ischemic patients who displayed a variable number of cardiovascular risk factors, and in whom heart disease was suspected.
| Prediction model | OR | 95% CI | Statistical significance |
|---|---|---|---|
| Model 1. EAT | 4.08 | 0.86, 19.37 | NS |
| Model 2. EAT + Type 2 diabetes mellitus | 9.90 | 0.96, 102.07 | NS |
| Model 3. EAT + MDA | 4.98 | 0.95, 26.22 | NS |
| Model 4. EAT + Type 2 diabetes mellitus + MDA | 11.55 | 1.03, 129.84 | P = 0.048 |
OR, odds ratio; CI, confidence interval; MDA, malondialdehyde.
NS, no statistically significant association (P > 0.05).
Discussion
Median EAT thickness in the present study was similar to values described in previous reports,5,7,8,11 and, as expected, was significantly higher in the patient subpopulation with greater cardiovascular risk (Framingham score ≥ 20). Members of this population were also associated with a particular profile that included older age, smoking, dyslipidaemia and ischemic heart disease. The EAT-associated increase in cardiovascular risk shown in the present study could be extrapolated to the general population because the characteristics and distribution of comorbidities, and the prevalence of risk factors in the present patient sample are similar to those reported for the general Mexican population according to the 2012 Mexican National Health and Nutrition Survey and the National Registry of Acute Coronary Syndromes.25,26 A higher level of clinically-detectable atherogenic risk, detected as significant differences in FMD and CIMT, was identified in patients with a Framingham score ≥20. Interestingly, this subgroup was likely to already display advanced-stage vascular damage because biomarkers including NO, sICAM-1 and MDA, which are thought to participate in the early mechanisms of endothelial dysfunction,27,28 were not significantly different from patients with Framingham score <20.
Nitric oxide, a biomarker of endothelial dysfunction, seemed to be associated with EAT thickness in the group of patients with Framingham score ≥20, while CIMT was correlated with EAT thickness globally, and there were significant differences in EAT thickness between patients stratified by age, smoking, dyslipidaemia, type 2 diabetes mellitus and Framingham score (Figure 1). Moreover, there was a slightly more significant correlation between NO and EAT thickness than CIMT and EAT thickness in patients with a Framingham score ≥20. Waist circumference and HDL-C were also found to be correlated with EAT thickness, and MDA was found to be correlated with EAT thickness in the whole patient cohort, but not in patient groups stratified by Framingham score ≥20 versus < 20. The present observations are in agreement with those previously reported for a Mexican population,11 and together with the particular findings regarding the relationships between EAT and metabolic variables (dyslipidaemia, type 2 diabetes mellitus, waist circumference, HDL-C), NO and CIMT, suggest that EAT thickness does reflect adiposity and may be useful to identify patients who display conventional cardiovascular risk factors in the early stage of vascular damage, when endothelial mechanisms that promote healthy vascular responses might still be functioning.
The role of EAT as an independent biomarker of endothelial dysfunction and its potential use in cardiovascular risk stratification has been described in a Turkish population that was grouped according to the presence of coronary artery disease.29 Similarly, the clinical relevance of NO has been demonstrated in other studies that have associated polymorphisms that affect the concentration of NO with a prognosis of mortality resulting from cardiac disease.30,31 The relationship between EAT and NO observed in the present study appeared to be associated with a population that also displayed specific cardiovascular risk factors. This finding further supports the notion that EAT may be a biomarker of endothelial dysfunction and demonstrates that it may have potential clinical utility for stratifying patients with cardiovascular risk factors according to atherogenic risk.
Finally, logistic regression modelling showed that the interaction between an EAT ≥ 6.5 and a CIMT ≥ 1 becomes statistically significant when type 2 diabetes mellitus and MDA are included in the model, suggesting that both variables influence the ability of EAT thickness to reflect subclinical atherogenesis.
The results of the present study may be limited by the low number of patients included. The sample size calculations, and prospective balancing of patients with cardiovascular risks, meant that the sample size was high enough to evaluate associations between pro-atherogenic factors and EAT thickness, however, additional relevant associations with other variables might have been missed. Potential selection bias may have arisen from the exclusion of patients who underwent surgical heart manipulation and patients with stroke. Additionally, the influence of drugs that can potentially affect adipose tissue metabolism was not weighted in the study. Translational studies investigating biomarkers of endothelial dysfunction and their associations with EAT are rare, and the present findings should, therefore, be replicated in larger studies. The prognostic significance of the present findings should be assessed in longitudinal studies.
In conclusion, NO, a biomarker of endothelial dysfunction, and conventional cardiovascular risk factors, were associated with EAT thickness. The specific association between EAT thickness and NO in the population that was stratified by Framingham score, as well as the difference in EAT thickness between the populations stratified by NO, but not by CIMT, suggests that EAT can be used to identify patients with conventional cardiovascular risk factors at different stages of vascular damage. Finally, EAT-associated subclinical atherogenesis, which was defined as CIMT ≥ 1, was further influenced by MDA and type 2 diabetes mellitus.
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest.
Funding
This research received financial support from the E-015 institutional program the CONACYT FOSSIS program.
References
- 1.Sacks HS, Fain JN, Cheema P, et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord 2011; 9: 433–439. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation 2006; 114: 1531–1544. [DOI] [PubMed] [Google Scholar]
- 3.Matloch Z, Kotulák T, Haluzík M. The role of epicardial adipose tissue in heart disease. Physiol Res 2016; 65: 23–32. [DOI] [PubMed] [Google Scholar]
- 4.Cappellano G, Uberti F, Caimmi PP, et al. Different expression and function of the endocannabinoid system in human epicardial adipose tissue in relation to heart disease. Can J Cardiol 2013; 29: 499–509. [DOI] [PubMed] [Google Scholar]
- 5.Topaloglu O, Sayki Arslan M, Turak O, et al. Three noninvasive methods in the evaluation of subclinical cardiovascular disease in patients with acromegaly: epicardial fat thickness, aortic stiffness and serum cell adhesion molecules. Clin Endocrinol (Oxf) 2014; 80: 726–734. [DOI] [PubMed] [Google Scholar]
- 6.Demir B, Cengiz H, Ungan I, et al. The relationship between epicardial adipose tissue thickness and oxidative stress parameters in patients with isolated polycystic ovary syndrome. Gynecol Endocrinol 2015; 31: 531–535. [DOI] [PubMed] [Google Scholar]
- 7.Kocaman SA, Durakoğlugil ME, Çetin M, et al. The independent relationship of epicardial adipose tissue with carotid intima-media thickness and endothelial functions: the association of pulse wave velocity with the active facilitated arterial conduction concept. Blood Press Monit 2013; 18: 85–93. [DOI] [PubMed] [Google Scholar]
- 8.Temiz A, Gökmen F, Gazi E, et al. Epicardial adipose tissue thickness, flow-mediated dilatation of the brachial artery, and carotid intima-media thickness: associations in rheumatoid arthritis patients. Herz 2015; 40(Suppl 3): 217–224. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 2008; 94: e7. [DOI] [PubMed] [Google Scholar]
- 10.Bilgic Gazioglu S, Akan G, Atalar F, et al. PAI-1 and TNF-α profiles of adipose tissue in obese cardiovascular disease patients. Int J Clin Exp Pathol 2015; 8: 15919–15925. [PMC free article] [PubMed] [Google Scholar]
- 11.Yañez-Rivera TG, Baños-Gonzalez MA, Ble-Castillo JL, et al. Relationship between epicardial adipose tissue, coronary artery disease and adiponectin in a Mexican population. Cardiovasc Ultrasound 2014; 12: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–1847. [DOI] [PubMed] [Google Scholar]
- 13.Ottolenghi A. Interaction of ascorbic acid and mitochondrial lipids. Arch Biochem Biophys 1959; 79: 355–363. [Google Scholar]
- 14.Moshage H, Kok B, Huizenga JR, et al. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 1995; 41: 892–896. [PubMed] [Google Scholar]
- 15.American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 2016; 39(Suppl 1): S13–S22. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7: 79–108. [DOI] [PubMed] [Google Scholar]
- 17.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011; 12: 167–205. [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol 2002; 39: 257–265. [DOI] [PubMed] [Google Scholar]
- 20.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008; 21: 93–111. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder S, Enderle MD, Ossen R, et al. Noninvasive determination of endothelium-mediated vasodilation as a screening test for coronary artery disease: pilot study to assess the predictive value in comparison with angina pectoris, exercise electrocardiography, and myocardial perfusion imaging. Am Heart J 1999; 138: 731–739. [DOI] [PubMed] [Google Scholar]
- 22.Simova I, Denchev S. Endothelial functional and structural impairment in patients with different degrees of coronary artery disease development. Heart Vessels 2008; 23: 308–315. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39. [DOI] [PubMed] [Google Scholar]
- 24.Yingchoncharoen T, Agarwal S, Popović ZB, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr 2013; 26: 185–191. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Escamilla R, Villalpando S, Shamah-Levy T, et al. Household food insecurity, diabetes and hypertension among Mexican adults: results from Ensanut 2012. Salud Publica Mex 2014; 56(Suppl 1): s62–s70. [DOI] [PubMed] [Google Scholar]
- 26.Borrayo-Sánchez G, Madrid-Miller A, Arriaga-Nava R, et al. Risk stratified in the national registry of acute coronary syndromes at the IMSS. Rev Med Inst Mex Seguro Soc 2010; 48: 259–264. [in Spanish, English abstract]. [PubMed] [Google Scholar]
- 27.Mazzone A, Cusa C, Mazzucchelli I, et al. Cigarette smoking and hypertension influence nitric oxide release and plasma levels of adhesion molecules. Clin Chem Lab Med 2001; 39: 822–826. [DOI] [PubMed] [Google Scholar]
- 28.Mollace V, Gliozzi M, Musolino V, et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int J Cardiol 2015; 184: 152–158. [DOI] [PubMed] [Google Scholar]
- 29.Kaya H, Ertaş F, Oylumlu M. Relation of epicardial fat thickness and brachial flow-mediated vasodilation with coronary artery disease. J Cardiol 2013; 62: 343–347. [DOI] [PubMed] [Google Scholar]
- 30.Azzam N, Zafrir B, Fares F, et al. Endothelial nitric oxide synthase polymorphism and prognosis in systolic heart failure patients. Nitric Oxide 2015; 47: 91–96. [DOI] [PubMed] [Google Scholar]
- 31.Martinelli NC, Santos KG, Biolo A, et al. Polymorphisms of endothelial nitric oxide synthase gene in systolic heart failure: an haplotype analysis. Nitric Oxide 2012; 26: 141–147. [DOI] [PubMed] [Google Scholar]