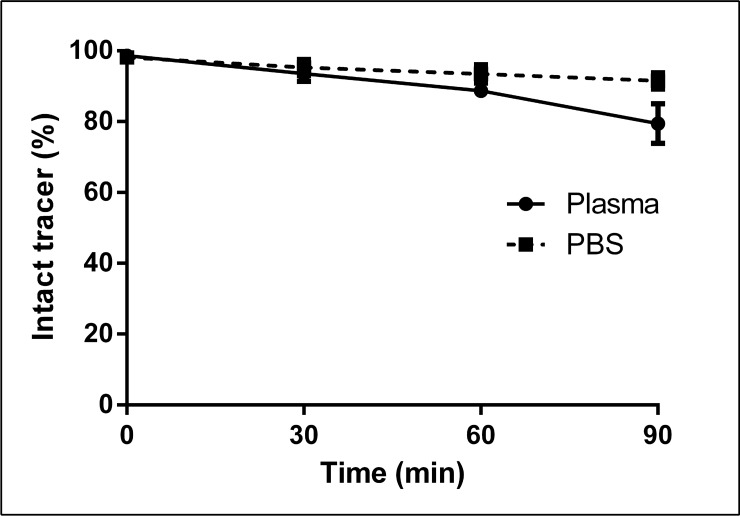
Figure 1. In-vitro stability of mutant [18F]FB-IL2v in rat plasma and PBS.
The radiopharmaceutical was incubated at 37°C for up to 90 min. The percentage of intact tracer was determined by the trichloroacetic acid precipitation assay. Data are presented as the mean of three independent experiments. Error bars represent the standard deviations.