Abstract
Recent advances in immunotherapy against cancer underscore the importance of T lymphocytes and tumor microenvironment, but few vaccines targeting cancer have been approved likely due in part to the dearth of common tumor antigens, insufficient immunogenicity and the evolution of immune evasion mechanisms during the progression to malignancy. Human papillomaviruses (HPV) are the primary etiologic agents of cervical cancer and progression from persistent HPV-infection to cervical intraepithelial lesions and eventually cancer requires persistent expression of the oncoproteins E6 and E7. This offers the opportunity to specifically target these virus-specific antigens for vaccine-induced clearance of infected cells before cancers develop. Here we have evaluated the immunogenicity of Adenovirus types 26 and 35 derived vectors expressing a fusion of HPV16 E6 and E7 oncoproteins after intramuscular and/or intravaginal immunization in mice. The adenovirus vectors were shown to transduce an intact cervicovaginal epithelium. Intramuscular prime followed by intravaginal boost maximized the induction and trafficking of HPV-specific CD8+ T cells producing IFN-γ and TNF-α to the cervicovaginal tract. Importantly, the cervicovaginal CD8+ T cells expressed CD69 and CD103, hallmarks of intraepithelial tissue resident memory CD8+ T cells. This prime/boost strategy targeting heterologous locations also induced circulating HPV-specific CD8+ T cell responses. Our study prompts further evaluation of intravaginal immunization with adenoviral vectors expressing modified E6 and E7 antigens for therapeutic vaccination against persistent HPV infection and cervical intraepithelial neoplasia.
Introduction
Cervical cancer is responsible for the death of 250,000 women each year and remains the third most common cancer in women worldwide1. Persistent Human papillomavirus (HPV) infection of the cervicovaginal mucosa with high-risk types is the central cause of cervical intraepithelial neoplasia of increasing severity (CIN1 to CIN3) and cervical cancer2. In addition, HPV infection causes a significant number of vulvar, penile, anal and oropharyngeal cancers. Transformation of cervicovaginal keratinocytes requires maintained expression of the oncoproteins E6 and E73. Most HPV infections are cleared naturally and a significant fraction of CIN2/3 lesions regress overtime4, 5. It has been proposed that infiltration of high grade lesions by mucosal T cells is a good predictor of lesion regression whereas exclusion of T cells from the lesions is associated with persistence of intraepithelial disease6. In this line it was shown that the programmed death-ligand 1 (PD-L1), an inhibitor of T cell receptor signaling, was up regulated even in low grade lesions (CIN1)7. These observations suggest that a therapeutic vaccine against persistent HPV infections and intraepithelial lesions should target viral antigens and induce cell-mediated immunity at the lesion site.
Three prophylactic VLP vaccines are used against HPV infections and prevent the occurrence of HPV-induced neoplasia8. They confer protection through the induction of neutralizing antibodies against the HPV capsid major protein L1. Once HPV persistent infection is established there is no evidence that these vaccines work in a therapeutic manner. In this context, incomplete vaccine coverage or acquisition of HPV infection before prophylactic vaccination will leave a significant number of women diagnosed with high-risk type HPV persistent infection and/or CIN. Promising results from therapeutic HPV vaccine clinical trials in suggest that a non-surgical alternative to the current treatments of high-grade lesions is achievable9, 10. In addition, a therapeutic vaccine could also provide a new intervention for women with persistent high-risk HPV infections and low-grade lesions and have a major public health impact in low resource settings when surgical procedures are unavailable.
Replication-defective viral vectors are attractive vehicles for genetic vaccination. They are intrinsically immunogenic, versatile platforms to encode various antigens, and may have a better safety profile than live attenuated viral vectors. Technologies based on adenoviruses (Ad) are the most advanced for genetic vaccination. Attractive features of Ad vectors are their ability to induce strong systemic T cell responses along with high serum antibodies after intramuscular (IM) immunization. Ad5 is the most common human serotype and despite highly prevalent type-specific neutralizing antibodies11, it has been historically the most utilized serotype as a vaccine vector. In spite of unsatisfactory results for HIV vaccination with Ad vector serotype 5 (Ad5) based vaccines12, technologies based on multiple Ad serotypes have shown promises for malaria, tuberculosis and Ebola virus vaccination13. Animal or less prevalent human Ad serotypes such as 26 (Ad26) and 35 (Ad35) have been developed to overcome type-specific anti-vector immunity induced by infection and/or vaccination14. Ad vectors have been used in numerous vaccine clinical trials alone against Ebola virus and tuberculosis or in prime/boost immunization regimens with other vaccine platforms, such as DNA or Modified Ankara virus (MVA) against HIV, malaria and respiratory syncytial virus13. Although Ad viruses are transmitted through mucosal sites (ocular, oral or respiratory) depending on the serotypes, the potential of Ad vectors for mucosal antigen delivery as vaccines remains to be explored in clinical settings.
Trafficking of CD8+ T cells to the cervical lesions is likely to be a critical parameter for success of a therapeutic HPV vaccine. However, with few exceptions, most clinical trials of HPV-therapeutic vaccines have evaluated vaccine immunogenicity in the blood compartment15. Tissue resident memory CD8+ T cells (Trm) cells have emerged recently as key players in antiviral immunity at mucosal surfaces and constitute a separate population from other circulating CD8+ T cell memory populations16, 17. In previous studies, we have shown that intravaginal (Ivag) prime/boost immunization with non-replicating HPV viral vectors was far superior to remote IM or intranasal immunizations with the vectors to induce high numbers of tissue resident memory CD8+ T cells in the cervicovaginal mucosa18, 19. In addition, Ivag immunization with HPV vectors was superior to confer protection in genital challenge with vaccinia virus or Herpes simplex virus. This increased propensity of Ivag immunization with HPV vectors to induce CD8 Trm is even more remarkable given the fact that IM immunization is far more potent than Ivag immunization to induce systemic and circulating CD8+ T cell responses.
Here we evaluated in mice a prototype therapeutic vaccine against HPV infection and neoplasia based on Ad26 and Ad35 expressing a fusion protein of HPV16 E6 and E7 oncoproteins (E6E7fus). We evaluated Ivag gene delivery with Ad26 and Ad35 vectors expressing reporter genes in time course and tropism experiments. We show that Ad vectors can transduce efficiently the intact cervicovaginal epithelium even in the presence of the potent HPV inhibitor carrageenan20. We evaluated prime/boost immunization regimens via heterologous routes combining classical IM immunization and Ivag immunization. We show that systemic prime followed by local Ivag booster is the most promising combination to efficiently induce concomitantly circulating and cervicovaginal resident CD8+ T cell responses. Furthermore, we show that Ad35 IM/Ad26 Ivag booster immunization induces E6 and E7 specific polyfunctional cytokine-secreting CD8+ T cells, but no detectable E6 or E7-specific CD4+ T cells, in the spleen and in the cervicovaginal mucosa.
Material and Methods
Viral vector design, production and characterization
E1/E3-deleted replication-deficient Ad26 and Ad35 expressing either GFP, Firefly luciferase, or HPV16 E6E7fus sequences under control of a CMV promoter were constructed, amplified, purified and characterized as described21, 22. Viral particle concentration was determined by optical density in the presence of SDS, and viral infectivity by TCID50 assay. Bioburden (MicroSafe, Millipore) levels and Endotoxin (MicroSafe, Millipore) levels met the preset release criteria for animal experiments.
Mice, immunization and sample preparation
C57Bl/6 and BALB/c female mice (8- to 10 weeks old) were purchased from Jackson laboratory and maintained in the animal care facilities at the National Cancer Institute (NCI) under specific-pathogen-free conditions. All procedures were reviewed and approved by the NCI Animal Care and Use Committee.
For IM immunization, 2.5×109 to 1×1010 Ad virus particles (vp) were diluted in 50ul PBS and injected in the quadriceps of anesthetized mice.
For Ivag instillation, mice were treated subcutaneously (SC) with 3mg Medroxyprogesterone acetate (Depoprovera) 5 days prior to instillation of viral vector. Five hours prior to instillation viral vector, mice were pretreated with 4% nonoxynol-9 (N-9) to expose the basement membrane or 2% carboxymethyl cellulose (CMC) to keep an intact epithelium. Ad viral vectors (2.5×109 to 1×1010 vp) were diluted in 2 % CMC or 1% ι-carrageenan, a potent topical HPV microbicide; and a total volume of 20ul was instilled intravaginally using a displacement pipette (Gilson).
A detailed description of the dissection procedure and samples preparation is available in supplementary methods.
In vivo bioluminescence assay
In vivo bioluminescence was monitored using a Xenogen IVIS Imager from 8hrs to 144hrs after Ivag instillation of Ad26 or Ad35 vectors expressing the firefly luciferase gene. Briefly, 3 min after Ivag instillation of 20μl D-Luciferin (Sigma, 15mg/ml), anesthetized mice were imaged using a 60sec exposure time and medium binning. Data are expressed as photon per sec (p/s).
Confocal microscopy
The cervicovaginal mucosa tissues were excised and embedded in OCT (Tissue-Tek) and kept frozen at −80C. In some experiments frozen 6-μm longitudinal sections of the cervicovaginal mucosa mounted on glass slides were stained with alexa-647 anti-MHC-II antibody with an antifade reagent (Prolong Gold with Molecular Probes) containing 4,6-diamidino-2-phenylindole (DAPI). Confocal images were acquired at the Confocal Microscopy Core Facility, Center for Cancer Research, National Cancer Institute, NIH, with Zeiss ZEN software on a Zeiss LSM 780 Confocal system using a 40X oil immersion objective and 364-nm, 488-nm, and 543-nm lasers. Images were analyzed for GFP expression using Adobe Photoshop, and color channel levels were adjusted uniformly across images.
In vitro T cell stimulation
For in vitro stimulation, cells were incubated for 6hrs at 37C, 5% CO2 in RPMI medium supplemented with 10% FBS, Sodium Pyruvate, L-Glutamine and 2-mercaptoethanol. Media containing overlapping 15mer peptide libraries of HPV16 E6, HPV16 E7 or the immunodominant peptide E749-57 peptide in the presence of brefeldin A (BD Golgi plug) were used to stimulate E6- and E7-specific cytokine production by T cells. Medium alone or containing PMA/ionomycin were used as a negative and positive stimulation controls.
Tetramer staining, intracellular cytokine staining and flow cytometry analysis
For tetramer staining, cell suspensions were stained for 30 min at 4°C with an APC-conjugated H-2Db/E749-57 tetramer (NIH Tetramer Core Facility, Atlanta, GA) and purified anti CD16/32 antibody to block FcR. The following fluorochrome-conjugated antibodies (Table S1) were added for another incubation 20 min at 4°C: Pacific Blue anti-CD3, APC-Cy7 anti-CD4, PE-Cy7 anti-CD44, PE-Cy7 anti-CD69 or PE-Cy7 anti-CXCR3, FITC anti-CD62L, PerCP-Cy5.5 anti-CD103, PE anti-CD127, and Pacific Orange anti-CD8.
For intracellular cytokine staining, cell suspensions were stained for 20 min with PE anti-CD8, PE.Cy7 anti-CD3, APC.Cy7 anti-CD4 and purified anti CD16/32 antibodies followed by viability assay (LIVE/DEAD Yellow, Invitrogen) and fixation in 4% paraformaldehyde. Intracellular staining using FITC anti-IFN-γ, PERCP.Cy5.5 anti-IL2, APC anti-TNF-α was done in permeabilization buffer (PermWash, BD) for 30min at 4C.
Adenovirus neutralization assay
In vitro adenovirus neutralization assay was performed as previously described 23.
Statistical analysis
Non-parametric Mann Whitney U test was performed to determine the P values in experiments with two independent groups. For experiments with more than two groups, a non-parametric one-way analysis (Kruskal-Wallis H test) was performed. Data are shown as mean ± SEM and P value ≤ to 0.05 (*), 0.01 (**), 0.001 (***) and 0.0001 (****) was considered statistically significant (ns: not significant).
Results
Ad26 and Ad35 transiently transduce the cervicovaginal mucosa epithelium upon N-9 treatment
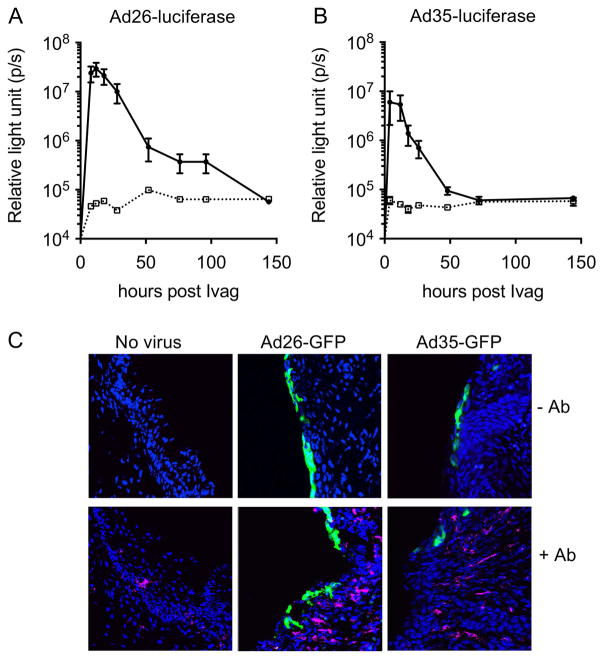
Few preclinical studies have examined delivery of Ad vectors (e.g. Ad5 serotype) through the vaginal route24, 25. We evaluated Ivag gene delivery with Ad vectors type 26 and 35 following a protocol developed for HPV pseudovirus genital infection26. This protocol requires disruption of the cervicovaginal epithelium with the spermicide N-9 to expose the cervicovaginal epithelium basement membrane and to facilitate access and binding of the viral particles. We reasoned that N-9 treatment prior to Ad vector Ivag instillation might also facilitate in vivo transduction and expression of genetically delivered antigens. Transduction of the cervicovaginal mucosa with Ad26 and Ad35 expressing luciferase was transient, peaked between 12hrs and 24hrs after vector instillation and returned to baseline by 48hrs (Fig. 1A, B). In spite delivering the same number of viral particles (2.5 × 109 vp), the peak luciferase signal was 20-times higher after Ad26 Ivag instillation compared to Ad35. Fluorescence imaging of the cervicovaginal mucosa at the peak of expression (16–24hrs) showed that the epithelium of the cervicovaginal mucosa was transduced with Ad26-GFP and to a lesser extent with Ad35-GFP (Fig. 1C). Immunofluorescence staining with MHC-II antibody rarely colocalized with GFP-positive cells suggesting predominant keratinocyte tropism of the Ad26 and Ad35 vectors (Fig. 1C).
Figure 1.
Intravaginal instillation of Ad vectors type 26 and 35 allows efficient gene delivery to the cervicovaginal mucosa epithelium. Depoprovera-treated mice were inoculated Ivag with N-9 followed 5 hours later with Ad vectors (2.5 × 109 vp) formulated in 2% carboxymethyl cellulose. Longitudinal in vivo bioluminescence imaging of the genital region of mice inoculated Ivag of with Ad26 (A, solid line) and Ad35 (B, solid line) vectors expressing firefly luciferase or without virus (A–B, dashed line). Data are expressed as mean relative light unit +/− SEM (A–B). For each time point (8hr to 144hr), 20ul of of 15mg/ml D-luciferin was instilled Ivag 3 min prior to imaging. Confocal microscopy imaging of GFP expression in the cervicovaginal mucosa epithelium after Ivag instillation of Ad26 and A35 vectors expressing GFP and immunofluorescent staining with (+Ab) or without (-Ab) Alexa-647 conjugated anti MHC-II antibody (C).
Prime/boost immunization via heterologous routes with Ad26 and Ad35 concomitantly induces cervicovaginal and systemic CD8+ T cell responses against HPV16 E7
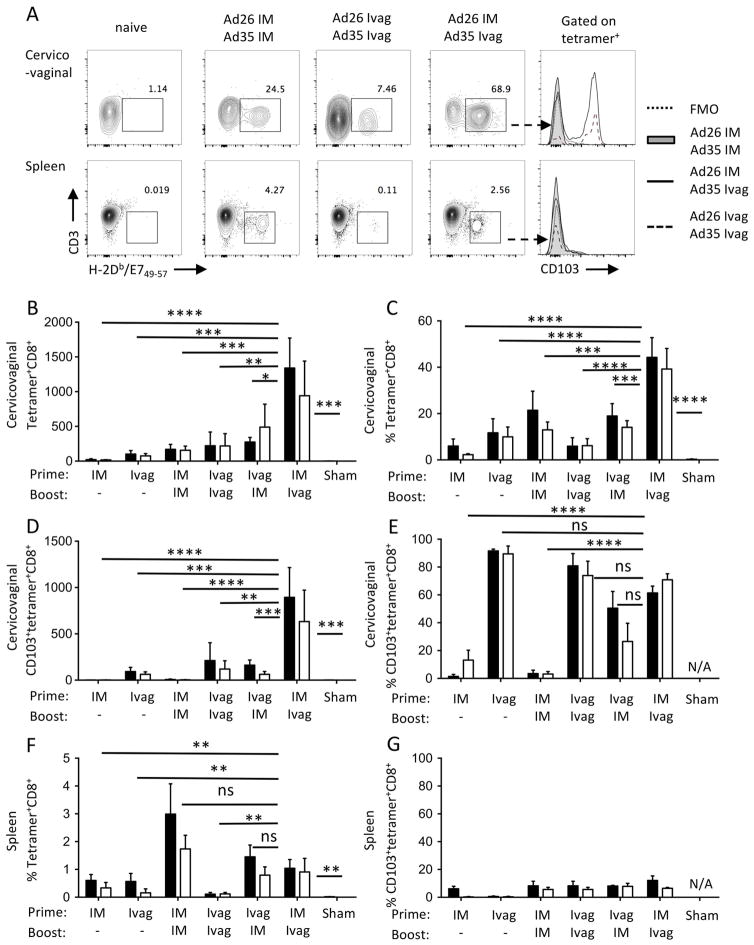
IM immunization is the most utilized route of administration of Ad vectors, with Ad5 being the most utilized serotype. We showed previously that in spite of the remarkable systemic immunogenicity of Ad5 vectors given IM, they are relatively ineffective at inducing cervicovaginal CD8+ T cell responses against a model viral antigen, in comparison to HPV vectors given Ivag19. Here, we evaluated Ad vectors prime/boost immunization via homologous or heterologous routes combining the classical IM route with the Ivag route. Specifically, we evaluated whether the vaccination route order influences the induction of cervicovaginal and systemic CD8+ T cell responses against the HPV16 E7 immunodominant epitope in C57Bl/6 mice. Because the induction of type-specific neutralizing antibodies against the vector capsid might impair booster immunization in mice, we alternated serotypes Ad26 and Ad35 for the prime and booster to allow comparison of route of administration without neutralizing antibodies interfering. HPV16 E7-specific CD8+ T cell responses were analyzed by flow cytometry using H2-DbE749-67 tetramers and CD103 integrin antibody, as a marker for intraepithelial lymphocytes and tissue resident lymphocytes at muco-cutaneous sites (Fig. 2A). The analysis was performed on pooled groups based on the route of administration rather than the serotypes used. Homologous prime/boost immunization Ivag/Ivag induced low numbers of cervicovaginal tetramer+CD8+ T cells that were comparable to IM/IM immunization (Fig. 2B). However, only Ivag/Ivag immunization induced expression of the integrin CD103 (Fig. 2E, P ≤ 0.0001). The frequency of E7-specific CD8+ T cells in the spleen was higher after IM/IM immunization compared to Ivag/Ivag immunization (P ≤ 0.0001), but they did not express CD103 (Fig. 2F and G).
Figure 2.
Prime boost immunization via heterologous routes with Ad vectors affects differentially the induction of systemic and cervicovaginal HPV-specific CD8+ T cell responses. Depoprovera-treated mice were immunized Ivag following N-9 pretreatment or IM with Ad vectors (2.5 × 109 vp) expressing a fusion protein of the HPV16 E6 and E7 oncoproteins. Mice (n= 5/group) were immunized sequentially one month apart with Ad26 for the prime and Ad35 for the boost (black histogram bars) and conversely with Ad35 for prime and Ad26 for boost (open histogram bars). Cervicovaginal (A–E) and spleen (A, F–G) cell suspensions were analyzed by flow cytometry for the presence of two weeks after immunization. HPV16 E7-specific CD8+ T cell responses are expressed as total number per organ (B) and percentage of CD8+ T cells (C and F). CD103 expression by HPV16 E7-specific CD8+ T cells is expressed as total number per organ (D) and percentage (E and G). Data are expressed as mean percentage or mean number of cells per organ ±SEM (N/A, not applicable). One-way analysis of variance and multiple comparisons to the sham-treated group (Kruskal-Wallis H test) was performed on groups based on the route of immunization.
Importantly, the order of route usage for heterologous prime/boost immunization was critical to induce cervicovaginal CD8+ T cell responses against HPV16 E7. Compared to IM/IM prime boost, mice primed IM and boosted Ivag displayed higher numbers (Fig. 2B, P ≤ 0.05) and percent of cervicovaginal E7-specific CD8+ T and cells (Fig. 2C, P ≤ 0.001 and Fig S1) but mice primed Ivag and boosted IM did not. The percentage of CD103 expression by E7-specific CD8+ T cells was increased in mice primed IM and boosted Ivag compared to IM/IM (P ≤ 0.001) but comparable if Ivag route was used for at least one immunization. The number of E7-specific CD8+ T cells expressing CD103 was increased in mice immunized IM/Ivag compared to Ivag/IM (P ≤ 0.001). The order of serotype usage did not play a significant role in the magnitude and tissue distribution of CD8+ T cell responses and in the expression of CD103 by E7-specific CD8+ T cells.
The order of the route of immunization but not the order of serotype usage affects differentially the induction of circulating CD8+ T cell responses
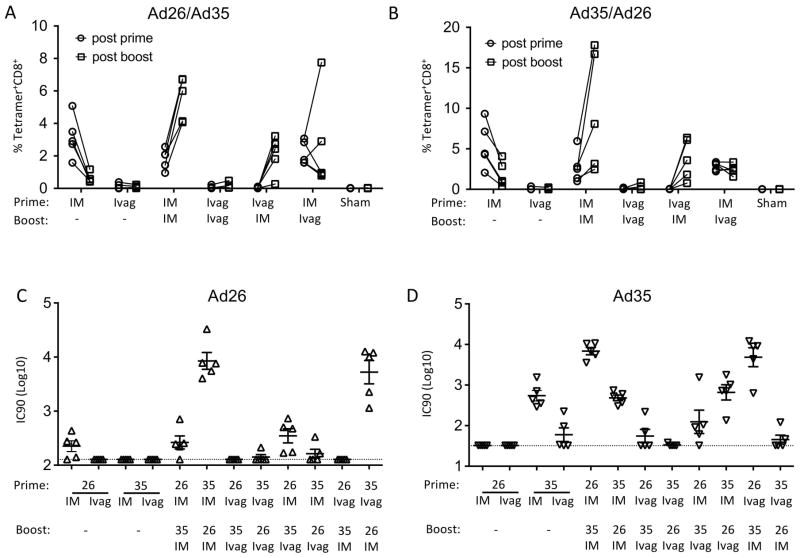
We next interrogated the impact of heterologous versus homologous prime/boost immunization on priming and recall of circulating E7-specific CD8+ T cell responses. Blood samples were collected 3 weeks after each immunization and, as seen in the spleen, the highest CD8+ T cell responses were observed in groups immunized at least once IM with either Ad26 or Ad35. After a single IM immunization with Ad26 or Ad35, the mean frequency of CD8+ T cells in blood was higher than after a single Ivag immunization but decreased by two-fold after a month (Fig. 3A–B). In IM primed animals, a single IM booster with Ad26 or Ad35 expanded the mean frequency of E7-specific CD8+ T cells whereas a single Ivag booster with Ad26 or Ad35 maintained or further enhanced the mean frequency of CD8+ T cells E7-specific. Together these data indicate that primary CD8+ T cell responses contracted and could be recalled by a secondary immunization. Also, these data indicate that IM immunization is more potent than Ivag immunization for priming and recall of circulating CD8+ T cell responses.
Figure 3.
The route of immunization with Ad vectors affects differentially the induction of circulating HPV16 E7-specific CD8+ T cells and adenovirus type-specific neutralizing antibodies. Depoprovera-treated mice were immunized Ivag following N9 pretreatment or IM with Ad vectors (2.5 × 109 vp) expressing a fusion protein of the HPV16 E6 and E7 oncoproteins. Mice were immunized sequentially one month apart with Ad26 for the prime and Ad35 for the boost (A) and conversely (B). All routes were tested alone by single immunization with Ad26 (A) or Ad35 (B). Blood samples were analyzed by H2-Db/E749-57 tetramer staining for the presence of HPV16 E7-specific CD8+ T cells in blood samples two weeks after immunization. Symbols connected by a line represent individual mice with open circles and open squares corresponding to post prime and post boost analysis, respectively (A, B). Adenovirus neutralizing antibody titers were measured in serum samples two weeks after the final immunization (C, D). Titers are expressed as mean (horizontal bar) and individual mice (symbols) for Ad26 (C, open triangle) and Ad35 (D, inverted open triangle).
Ivag immunization induces lower type-specific Ad neutralizing antibody titer compared to IM immunization
We evaluated neutralizing antibody titers against Ad26 and Ad35 in plasma samples collected three weeks after the booster immunization. Ivag immunization with either Ad26 or Ad35 induced low to no type-specific neutralizing antibody titers whereas IM immunization with either Ad26 or Ad35 induced high type-specific neutralizing antibody titers (Fig 3C, D). These data suggest that repeated Ivag immunization might be achieved using the same serotype. No detectable cross-neutralizing antibodies were measured upon a single immunization with Ad26 or Ad35, IM or Ivag. Interestingly the neutralizing antibody titers against the type used for priming were lower compared to the neutralizing antibody titers against the type used for booster which might be due to early decrease in vector-specific antibody responses upon a single exposure.
Ad26 and Ad35 transduce intact cervicovaginal mucosa epithelium and are partially inhibited by carrageenan
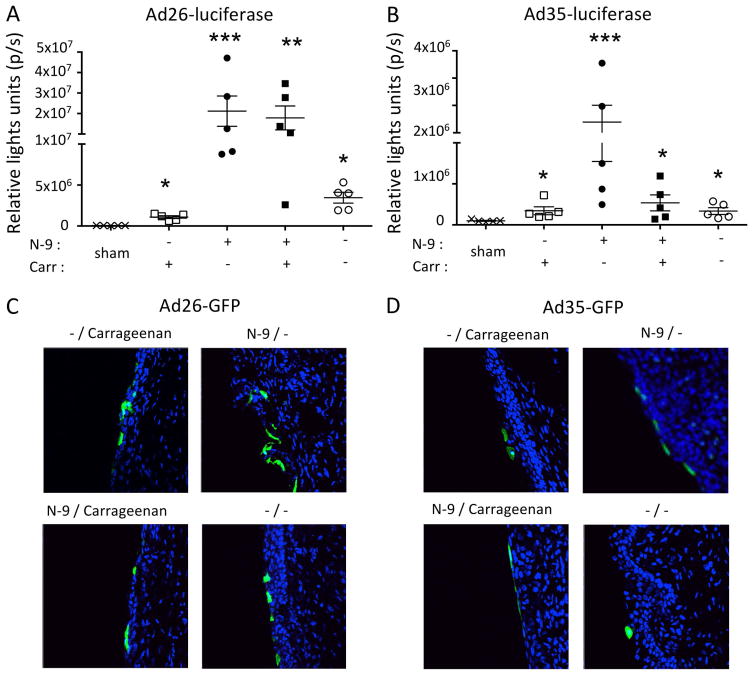
The use of N-9 in our topical therapeutic vaccination procedure might transiently potentiate HPV reinfection through autoinoculation or from an infected sexual partner 27. Therefore, we evaluated the ability of Ad vectors to transduce an intact cervicovaginal epithelium or to transduce the cervicovaginal epithelium in the presence of carrageenan, a sulfated polysaccharide that is a potent HPV microbicide 20. We used Ad vectors expressing luciferase for quantification of in vivo transduction and GFP to analyze tropism at the peak of expression as determined in previous experiments (Fig. 1). Although disruption of the cervicovaginal epithelium increased significantly in vivo transduction by Ad26 and Ad35 both types were able to transduce an intact cervicovaginal epithelium. Surprisingly, carrageenan had only a modest effect on Ad26- and Ad35-mediated transduction, respectively, which suggests that glycosaminoglycans play a minor role in cell attachment and infection by these Ad vectors types (Fig. 4A, B). Interestingly, the preferential tropism for the epithelial layers of the cervicovaginal mucosa was conserved across all conditions tested (Fig 4C, D).
Figure 4.
Effect of N-9 and ι-carrageenan on Ad vector type 26 and 35 transduction of the cervicovaginal mucosa. Depoprovera-treated mice (n=5/group) were inoculated Ivag with or without N-9 followed 5 hours later with Ad vectors (2.5 × 109 vp) given in CMC or i-carrageenan. Longitudinal in vivo bioluminescence imaging of the genital region of mice inoculated Ivag of with Ad26 (A) and Ad35 (B) vectors expressing firefly luciferase. Data are expressed as mean +/− SEM (A–B). For each time point (18hrs), 20ul of of 15mg/ml D-luciferin was instilled Ivag 3min prior to imaging. Confocal microscopy imaging of GFP expression in the cervicovaginal mucosa epithelium after Ivag instillation of Ad26 (C) and A35 (D) vectors expressing GFP. One-way analysis of variance and multiple comparisons (Kruskal-Wallis H test) was performed to determine statistical significance.
Ad26 immunogenicity is retained upon Ivag instillation on intact cervicovaginal epithelium and induces preferentially CD69+CD103+ tissue resident CD8+ T cells
We evaluated immunogenicity after Ivag priming with Ad26-E6E7fus vector on an intact or disrupted cervicovaginal epithelium either in the absence or presence of carrageenan (Fig. 5). We focused on Ad26 vectors for Ivag immunization because it transduced more efficiently the cervicovaginal epithelium than Ad35 (Fig. 1A, B).
Figure 5.
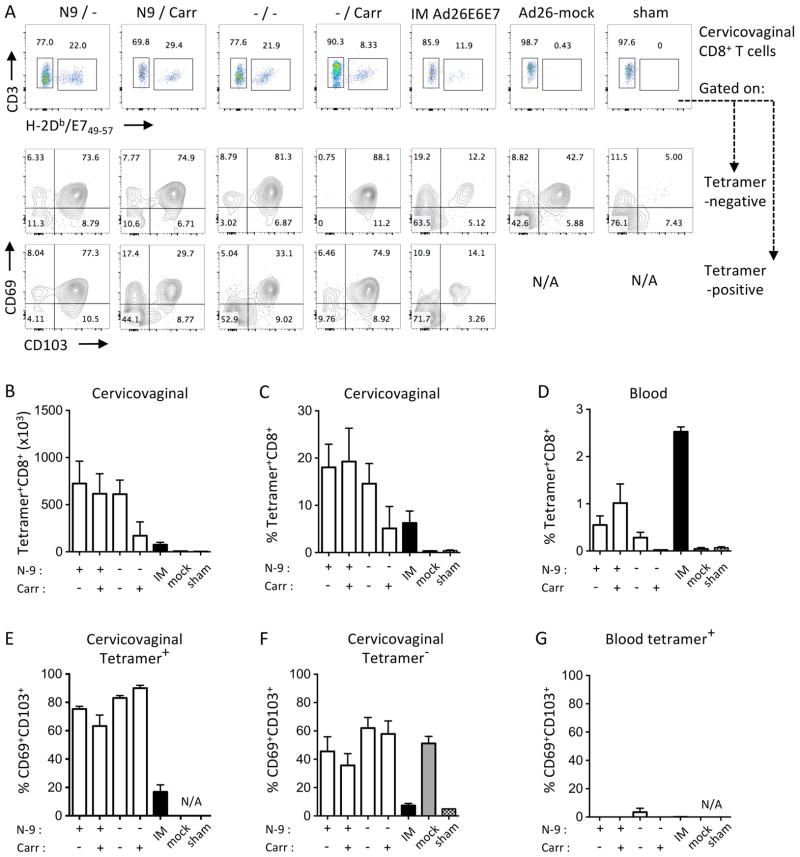
Effect of N-9 and ι-carrageenan on Ad26 immunogenicity after Ivag priming. Depoprovera-treated mice (n=5/group) were inoculated Ivag with or without N-9 followed 5 hours later with Ad26-E6E7fus (1.0 × 1010 vp) delivered in CMC or ι-carrageenan. Additional groups, IM Ad26-E6E7fus (IM), Ivag Ad26-mock with N-9 and no carrageenan (mock) and Ivag N-9 without carrageenan (sham) were used as control. Cervicovaginal (A–C, E–F) and blood samples (D–G) were analyzed by H2-Db/E749-57 tetramer staining for the presence of HPV16 E7-specific CD8+ T cells three weeks after immunization. Representative FACS plot for H2-Db/E749-57 tetramer staining on cervicovaginal CD8+ T cells (A, row1) and for CD69 and CD103 expression by tetramer negative (A, row2) and tetramer-positive (A, row3). Data are expressed as mean percentage or mean number of cells per organ ± SEM (N/A, not applicable).
We used 1 × 1010 vp of Ad26-E6E7fus vector for Ivag priming to induce consistent primary CD8+ T cell responses. Mice were pre-treated Ivag with 4% N-9 or with 4% CMC as a control for intact epithelium. Five hours later, Ad26-E6E7fus was diluted in 1% ι-carrageenan or 2% CMC as a control and delivered Ivag. Three weeks after priming, we quantified cervicovaginal HPV16 E7-specific CD8+ T cells by flow cytometry using H2-DbE749-67 tetramers (Fig. 5A, row 1). We assessed the expression of CD69 and CD103 markers to determine the tissue resident memory phenotype of E7-tetramer-negative (Fig. 5A row 2) and E7-tetramer-positive (Fig. 5A row 3) cervicovaginal CD8+ T cells. Interestingly, Ivag priming after N-9 treatment induced the same frequency and number of E7-specific CD8+ T cells with or without carrageenan, consistent with our in vivo transduction data (Fig. 4). Surprisingly, Ivag priming on an intact epithelium induced similar frequency and number of cervicovaginal E7-specific CD8+ T cells to N-9 treated mice despite reduced in vivo transduction efficacy compared to Ivag immunization after N-9 pretreatment (Fig. 5A–C). Carrageenan decreased the induction of E7-specific CD8+ T cell after Ivag priming on an intact epithelium which correlates with lowest in vivo transduction (Fig. 5A–E). All Ivag immunization procedures induced cervicovaginal E7-specific CD8+ T cells with a tissue resident memory phenotype characterized by CD69 and CD103 expression (Fig, 5E). Interestingly, CD8+ tetramer negative fraction was also enriched for Trm cells in all groups immunized Ivag with Ad26 vectors including the group immunized with a Ad26-empty vector but not groups immunized IM with Ad26-E6E7fus or sham immunized (Fig. 5F). These data suggest that Ivag immunization with Ad26 might induce a strong anti-vector CD8+ T cell response. Finally, all Ivag immunization procedures induced circulating E7-specific CD103-CD69-CD8+ T cells albeit of lower magnitude than a single IM immunization (Fig. 5D, G).
Ad26-E6E7fus Ivag booster immunization on intact cervicovaginal epithelium induces a broad polyfunctional CD8+ T cells response against the HPV16 E6 and E7 antigens
Next, we evaluated the CD8+ T cells cytokine response after IM prime with Ad35-E6E7fus followed by booster Ivag immunization with Ad26-E6E7fus vector onto intact or disrupted cervicovaginal epithelium (+/− N-9) with or without the addition of carrageenan (+/− Carr). As immunization controls some mice that were primed IM with Ad35-E6E7fus were then Ivag immunized with Ad26-mock pretreated with N-9 (mock) or treated with N-9 only (sham); or primed IM with Ad35-mock and immunized later Ivag with Ad26-E6E7fus with N-9 (Ad26-E6E7); or primed IM with PBS (sham) and later treated with N-9 only (sham). Spleen and cervicovaginal single cell suspensions were incubated with E6 or E7 overlapping peptide mix, E749-67 peptide, PMA/ionomycin as a positive control or in medium only as negative control. IFN-γ production by CD8+ T cells was measured by intracellular cytokine staining as shown in Figure 6A.
Figure 6.
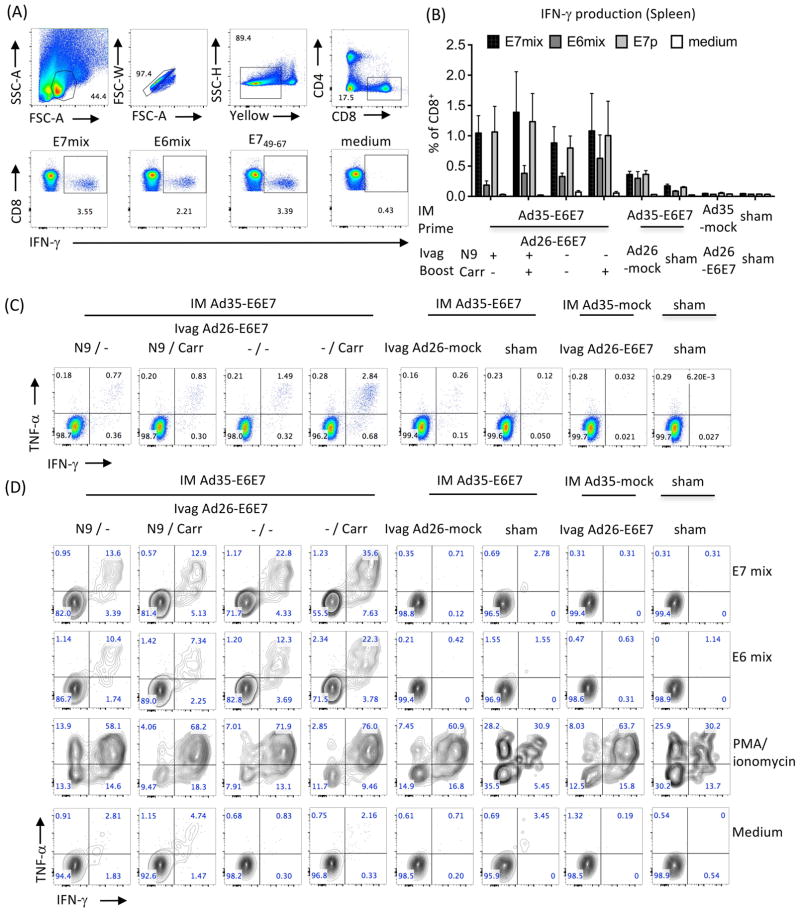
Effect of N-9 and ι-carrageenan on the induction of cervicovaginal and systemic polyfunctional CD8+ T cells after Ivag booster immunization with Ad26-E6E7fus. Mice (n=5/group) were immunized IM with Ad35-E6E7fus (1.0 × 1010 vp), Ad35-mock (1.0 × 1010 vp) or PBS as control. One month later, Depoprovera-treated mice were inoculated Ivag with or without N-9 (+/−) treatment followed 5 hours later with Ad26-E6E7fus (1.0 × 1010 vp) delivered in CMC or ι-carrageenan (+/−). As controls, some groups primed IM with Ad35-E6E7fus were immunized later Ivag with Ad26-mock (1.0 × 1010 vp) with N-9 (mock) or treated with N-9 only (sham); primed IM with Ad35-mock were immunized one month later Ivag with Ad26-E6E7fus following treatment with N-9 (Ad26-E6E7); additional controls include mice primed IM with PBS (sham) and treated one month later with with N-9 only (sham). Three weeks after booster immunization, cytokine production by spleen (A, B) and cervicovaginal (C, D) CD8+ T cells was assessed by intracellular cytokine staining after in vitro stimulation with overlapping peptide mixes of E7 (black bars in B) or E6 (dark grey bars in B) E749-67 (light grey bars in B), medium only (white bars in B) or PMA/ionomycin. FACS plot showing the gating strategy and analysis of IFN-g production by splenic CD8+ T cells (A, B). FACS plot presentative of IFN-γ and TNF-α production by splenic (individual samples) and cervicovaginal (pooled samples) CD8+ T cells (C, D). Data are expressed as mean percentage of cells ± SEM (5 mice per group).
E7-specific IFN-γ production by CD8+ T cells in the spleen was observed in all groups primed IM with Ad35-E6E7fus (Fig. 6B) but was higher in groups receiving a booster Ivag immunization compared to group not receiving a booster immunization. Interestingly, an intact or disrupted epithelium with or without carrageenan did not impact the recall of systemic CD8+ T cell responses after Ivag immunization (Fig. 6B). Importantly, in vitro stimulation of spleen cells with the minimal immunodominant epitope E749-67 peptide induced similar frequency of IFN-γ+CD8+ T cells compared to the E7 peptides mix, which suggests that most E7-specific CD8+ T cells reacted to the E749-67 immunodominant epitope. E6-responding IFN-γ+CD8+ T cells were detected in the spleen although at lower level than E7-specific IFN-γ+CD8+ T cells. Most spleen HPV-specific CD8+ T cells induced after IM Ad35-E6E7fus immunization were polyfunctional producing simultaneously IFN-γ and TNF-α (Fig. 6C) and were expanded by Ivag booster immunization with Ad26-E6E7fus.
Due to limited number of cells extracted from the cervicovaginal mucosa, we performed each in vitro stimulation condition on pooled single cell suspensions of groups of 5 mice. All groups primed IM with Ad35-E6E7fus and boosted Ivag with Ad26-E6E7fus displayed high frequency of polyfunctional CD8+ T cells producing IFN-γ and TNF-α simultaneously in E6 and E7 overlapping peptide mix in vitro stimulation (Fig. 6D). Ivag booster immunization with Ad26-mock vector did not redirect circulating E6/E7-specific CD8+ T cells induced by IM prime with Ad35-E6E7fus to the vaginal tract (Fig. 6D). However, all groups receiving Ivag Ad26-E6E7fus or Ad26-mock displayed a high frequency of polyfunctional CD8+ T cells upon stimulation with PMA/ionomycin compared to sham treated groups. These data strongly suggest that Ivag immunization with Ad vectors triggers vector-specific CD8+ T cell responses.
Analysis of cervicovaginal CD4+ T cell responses after IM/Ivag prime-boost immunization with Ad35 and Ad26 vectors
Using E6 and E7 15mer overlapping peptides for in vitro stimulation, we did not detect E6 or E7-specific CD4+ T cells producing IFN-γ or TNF-α (Fig S2 and data not shown). All groups boosted Ivag with Ad26-E6E7fus showed infiltration of the cervicovaginal mucosa by CD4+ T cells (Fig S2B). Most infiltrating CD4+ T cells expressed CD69 but not CD103 suggesting a submucosal resident phenotype (Fig S2C). Interestingly, in all groups immunized Ivag with Ad26-E6E7fus, PMA/ionomycin restimulation induced higher CD4+ T cells IFN-γ and TNF-α production than in sham-treated groups (Fig S2D). Increased CD4+ T cells infiltration and cytokine production was also observed in mice boosted Ivag with Ad26-mock compared to sham-treated suggesting induction of adenovirus-specific CD4+ T cells (Fig S2). Next, we assessed the role of CD4+ T cells in the induction of CD8+ T cells after Ad26-E6E7fus Ivag immunization (Fig S3A–B). Antibody mediated CD4-depletion prevented the induction of tetramer+CD8+ T cell responses in blood and cervicovaginal mucosa and suppressed the induction of E7-specific IFN-γ+TNF-α+ CD8+ T cells in spleen (Fig S3C–E).
Discussion
Ad26 and Ad35 were developed as alternative types to Ad5 to circumvent vector-specific neutralizing antibodies for prime-boost immunization. Clinical grade Ad26 and Ad35 have been mostly utilized for systemic delivery, with a few exceptions for intranasal delivery. Preclinical evaluation of Ad vectors indicates that the quality of the memory CD8+ T cells elicited after IM immunization varies depending on the Ad serotype28. These differences in immunogenicity might result from altered antigen expression due to differences in cellular tropism and receptor usage or altered modulation of innate cytokine responses between Ad serotypes29.
In this study, we show that Ad26 and Ad35 vectors transduce the intact mouse cervicovaginal epithelium, whereas HPV pseudovirions, used for Ivag immunization, require epithelium permeabilization or disruption to infect basal cells of pluristratified epithelia27. Such difference might be due to the expression of Ad specific receptors and attachment factors at the apical side of epithelial cells. Also, Ad fiber proteins have been shown to increase airway epithelium permeability through binding to the Coxsackievirus and Adenovirus Receptor (CAR), resulting in disruption of tight junctions and spread of viral particles to the lumen30. A similar mechanism might facilitate transduction of the epithelial cells upon Ivag instillation. Carrageenan inhibition experiments indicate that glycosaminoglycans may play a limited role in cell attachment and infection by Ad26 and Ad35 vectors, consistent with previous studies with Ad2 and Ad531. Ad vectors tropism might differ between human and mice due to differential expression of receptors such as CAR or CD46 and might constitute a limitation to our preclinical evaluation 32. However, because mucosal epithelia are the natural sites of infection by human Ad, it is expected that Ad vectors should retain their strong ability to transduce the cervicovaginal epithelium in women.
Few preclinical studies have evaluated Ivag delivery of a vaccine for treatment of HPV-induced neoplasia. Ongoing clinical trials for therapeutic treatment of CIN are introducing the use of topical TLR agonists such as Imiquimod to subvert the immune suppressive environment, promote immunogenic cell death and recruit HPV-specific T cells33. To date, impressive clinical response has been observed in a large clinical trial involving multiple rounds of intralesional injection of an MVA vector expressing bovine papillomavirus E2 protein34. Ongoing clinical trials are combining vaccination with topical Imiquimod to promote trafficking of antigen-specific T cells to the lesion (NCT00788164, NCT00988559). Together these studies underscore the potential of therapeutic interventions targeting the lesion site.
Our results challenge the notion that the cervicovaginal mucosa is poorly immunogenic. We provide evidence for the first time that Ivag vaccination with Ad26-E6E7fus or Ad35-E6E7fus increases trafficking of HPV-specific CD8+ T cells to the cervicovaginal mucosa. Our results extend previous studies showing immunogenicity and protection after Ivag delivery of Ad5 expressing HIV-1 GAG or HSV-2 gB antigens in preclinical infection models25, 35. Our immunization strategy provides a way to amplify local CD8+ T cell responses by IM priming immunization followed by Ivag boosting. Local antigen presentation of E6 and E7 antigens might explain the preferential induction of cervicovaginal Trm CD8+ T cells compared to sole IM immunization. Such presentation might trigger the recruitment of preexisting circulating memory CD8+ T cells, as demonstrated in the context of genital viral infection36. Local proliferation might also explain the expansion of HPV-specific CD8+ T cells as shown after Ivag priming or boosting with other viral vectors19, 37.
The fact that primary CD8+ T cell responses depend on the presence of CD4+ T cells after Ivag immunization with Ad26 vectors is concordant with the requirement of CD4+ T cell help in the formation and maintenance of memory CD8+ T cells after IM immunization38. We did not detect CD4+ T cell responses against the E6E7fus antigen after restimulation with E6 and E7 15mer peptide mixes which should have allowed to detect most CD4+ T cells specific of classical MHC-II restricted peptides39. Therefore, it is possible that CD4+ T helper cells instead react to adenoviral antigens. CD4+ T cell responses against HPV16 E6 and E7 antigens are known to be weak in the C57BL/6 strain of mice, which harbors only one MHC-II molecule, and so may be suboptimal for induction of CD8+ T cell responses40. We speculate that such E6- and E7-specific CD4+ T cell responses might be easier to elicit in outbred human populations with higher diversity and polymorphisms of MHC-II molecules.
Our study provides evidence that Ivag vaccination with Ad26 or Ad35 vectors induces high number of CD8+ Trm cells in the cervicovaginal mucosa. CD8+ Trm are excluded from the circulation which prevents their monitoring in blood samples in the context of infections or vaccination. Seminal studies in humans have described the anatomical distribution of memory CD8+ T cells including Trm and validated CD103 and CD69 as markers of CD8+ Trm at body surfaces 41, 42. However, the precise contribution of CD8+ Trm to spontaneous clearance of HPV persistent infection or regression of CIN has not yet been studied. Local amplification of CD8+ Trm might overcome early immune escape mechanisms and shift the ratio of effector CTL/target cells in favor of improved clearance. Finally, local induction of vector-specific CD8+ Trm might trigger bystander activation of CD8+ Trm and could lead to tissue conditioning to an anti-viral state and promote virological clearance or lesion regression. Such bystander effects have been described with viral vectors or peptides against CIN or viral infections34, 43–45.
Accumulating evidence suggests that, early upon infection, lesions evolve toward an immunosuppressive state as shown by up regulation of PD-L1, exclusion of T cells from infiltrating the lesion, and/or down regulation of MHC molecules. The current syngeneic mouse orthotropic models for HPV-associated cancer are associated with rapid tumor growth, do not recapitulate the slow progression of CIN46, 47 and prevent the evaluation of our prime/boost vaccination regimen. The primary goal of this study was to evaluate immunogenicity of Ad vectors given Ivag in a prime/boost vaccination regimen, in particular CD8+ T cells trafficking to the cervicovaginal epithelium. IM/Ivag immunization was by far the best combination to induce simultaneous production of IFN-γ and TNF-α by E6- and E7-specific CD8+ T cells and was higher in the cervicovaginal mucosa compared to secondary lymphoid organs. Production of both cytokines is the best indication that the cervicovaginal CD8+ T cells are functional and endowed with potentially anti-viral and anti-tumoral activities48. In addition to direct tumoricidal activities, local production of IFN-γ and TNF-α could increase expression of MHC molecules by transformed cells and render them susceptible to CD8+ T cells-mediated cytolysis.
The feasibility of translating our vaccination strategy to treat cervical and vaginal intraepithelial neoplasia and persistent high-risk HPV infection in women should be evaluated. Several clinical trials involving intravaginal delivery of drugs or microbicides support the conclusion that self-administration of gel-based vaginal vaccine might be well accepted and tolerated49, 50. We believe that a self-administered adenoviral vaccine could be a critical component of a single clinic visit screen-and-treat public health program to lower cervical cancer rates in mid-adult women in low resource settings. In this scenario, a positive result in a point-of-care HPV test of a cervicovaginal sample would trigger an Ad IM prime vaccination in positive women followed several weeks later by an in-home Ad Ivag booster immunization. This strategy might induce the accumulation of intralesional E6- and E7-specific CD8+ Trms, overcome local immune suppression in early and advanced lesions and promote high rates of virological clearance and/or CIN regression.
Supplementary Material
Acknowledgments
The authors thank the NIH Tetramer Core facility for synthesis of the H-2Db-E749-57 tetramer. S. Khan, J. Vellinga, R. Zahn and G. Scheper are employees of Janssen Pharmaceutical Companies.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–5. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25:215–22. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM, Group A. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 5.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, Wilgus B, Yutzy W, Daniel R, Shah K, Peng S, Hung C, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11:4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, Kos F, Teague J, Jiang Y, Barat NC, Jungbluth AA. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594–602. doi: 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 8.Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat Rev Microbiol. 2012;10:681–92. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. The New England journal of medicine. 2009;361:1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 10.Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, Edwards L, Parker RL, Denny L, Giffear M, Brown AS, Marcozzi-Pierce K, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JA, Barouch DH, Baden LR. Nonreplicating vectors in HIV vaccines. Curr Opin HIV AIDS. 2013;8:412–20. doi: 10.1097/COH.0b013e328363d3b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: from immunogenicity to impact. Curr Opin Immunol. 2016;41:47–54. doi: 10.1016/j.coi.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Gordo E, Podgorski II, Downes N, Alemany R. Circumventing antivector immunity: potential use of nonhuman adenoviral vectors. Hum Gene Ther. 2014;25:285–300. doi: 10.1089/hum.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. 2014;6:221ra13. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21:688–97. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Cuburu N, Wang K, Goodman KN, Pang YY, Thompson CD, Lowy DR, Cohen JI, Schiller JT. Topical herpes simplex virus 2 (HSV-2) vaccination with human papillomavirus vectors expressing gB/gD ectodomains induces genital-tissue-resident memory CD8+ T cells and reduces genital disease and viral shedding after HSV-2 challenge. J Virol. 2015;89:83–96. doi: 10.1128/JVI.02380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuburu N, Graham BS, Buck CB, Kines RC, Pang YY, Day PM, Lowy DR, Schiller JT. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122:4606–20. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, Thierry AC, Mayor C, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis. 2016;16:311–20. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 22.Khan S, Oosterhuis K, Wunderlich K, Bunnik EM, Bhaggoe M, Boedhoe S, Karia S, Steenbergen RDM, Bosch L, Serroyen J, Janssen S, Schuitemaker H, et al. Development of a replication-deficient adenoviral vector-based vaccine candidate for the interception of HPV16- and HPV18-induced infections and disease. Int J Cancer. 2017;141:393–404. doi: 10.1002/ijc.30679. [DOI] [PubMed] [Google Scholar]
- 23.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–52. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Zhang M, Zhou C, Zhao X, Iijima N, Frankel FR. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol. 2008;180:2504–13. doi: 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Q, Thomson CW, Rosenthal KL, McDermott MR, Collins SM, Gauldie J. Immunization with adenovirus at the large intestinal mucosa as an effective vaccination strategy against sexually transmitted viral infection. Mucosal Immunol. 2008;1:78–88. doi: 10.1038/mi.2007.3. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JN, Kines RC, Katki HA, Lowy DR, Schiller JT. Effect of Pap smear collection and carrageenan on cervicovaginal human papillomavirus-16 infection in a rhesus macaque model. J Natl Cancer Inst. 2011;103:737–43. doi: 10.1093/jnci/djr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–61. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 28.Penaloza-MacMaster P, Alayo QA, Ra J, Provine NM, Larocca R, Lee B, Barouch DH. Inhibitory receptor expression on memory CD8 T cells following Ad vector immunization. Vaccine. 2016;34:4955–63. doi: 10.1016/j.vaccine.2016.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teigler JE, Iampietro MJ, Barouch DH. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J Virol. 2012;86:9590–8. doi: 10.1128/JVI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walters RW, Freimuth P, Moninger TO, Ganske I, Zabner J, Welsh MJ. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell. 2002;110:789–99. doi: 10.1016/s0092-8674(02)00912-1. [DOI] [PubMed] [Google Scholar]
- 31.Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75:8772–80. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wold WS, Ison MG. Adenovirus. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Philadelphia: LIPPINCOTT WILLIAMS & WILKINS; 2013. p. 1732. [Google Scholar]
- 33.Cuburu N, Schiller JT. Moving forward with human papillomavirus immunotherapies. Hum Vaccin Immunother. 2016:1–6. doi: 10.1080/21645515.2016.1199302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosales R, Lopez-Contreras M, Rosales C, Magallanes-Molina JR, Gonzalez-Vergara R, Arroyo-Cazarez JM, Ricardez-Arenas A, Del Follo-Valencia A, Padilla-Arriaga S, Guerrero MV, Pirez MA, Arellano-Fiore C, et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum Gene Ther. 2014;25:1035–49. doi: 10.1089/hum.2014.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza AP, Haut LH, Silva R, Ferreira SI, Zanetti CR, Ertl HC, Pinto AR. Genital CD8+ T cell response to HIV-1 gag in mice immunized by mucosal routes with a recombinant simian adenovirus. Vaccine. 2007;25:109–16. doi: 10.1016/j.vaccine.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–13. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Sui Y, Kato S, Hogg AE, Steel JC, Morris JC, Berzofsky JA. Vaginal type-II mucosa is an inductive site for primary CD8(+) T-cell mucosal immunity. Nat Commun. 2015;6:6100. doi: 10.1038/ncomms7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provine NM, Larocca RA, Penaloza-MacMaster P, Borducchi EN, McNally A, Parenteau LR, Kaufman DR, Barouch DH. Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses. J Immunol. 2014;192:5214–25. doi: 10.4049/jimmunol.1302806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones EY, Fugger L, Strominger JL, Siebold C. MHC class II proteins and disease: a structural perspective. Nat Rev Immunol. 2006;6:271–82. doi: 10.1038/nri1805. [DOI] [PubMed] [Google Scholar]
- 40.Duarte N, Penha-Goncalves C. The MHC locus controls size variations in the CD4 compartment of the mouse thymus. Immunogenetics. 2001;53:662–8. doi: 10.1007/s00251-001-0377-9. [DOI] [PubMed] [Google Scholar]
- 41.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, Kato T, Farber DL. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–97. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woon HG, Braun A, Li J, Smith C, Edwards J, Sierro F, Feng CG, Khanna R, Elliot M, Bell A, Hislop AD, Tangye SG, et al. Compartmentalization of Total and Virus-Specific Tissue-Resident Memory CD8+ T Cells in Human Lymphoid Organs. PLoS Pathog. 2016;12:e1005799. doi: 10.1371/journal.ppat.1005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–5. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 44.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Tarbet EB, Toro H, Tang DC. Adenovirus-vectored drug-vaccine duo as a potential driver for conferring mass protection against infectious diseases. Expert Rev Vaccines. 2011;10:1539–52. doi: 10.1586/erv.11.141. [DOI] [PubMed] [Google Scholar]
- 46.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 47.Decrausaz L, Goncalves AR, Domingos-Pereira S, Pythoud C, Stehle JC, Schiller J, Jichlinski P, Nardelli-Haefliger D. A novel mucosal orthotopic murine model of human papillomavirus-associated genital cancers. Int J Cancer. 2011;128:2105–13. doi: 10.1002/ijc.25561. [DOI] [PubMed] [Google Scholar]
- 48.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, Ranta F, Ullrich S, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 49.Friedland BA, Hoesley CJ, Plagianos M, Hoskin E, Zhang S, Teleshova N, Alami M, Novak L, Kleinbeck KR, Katzen LL, Zydowsky TM, Fernandez-Romero JA, et al. First-in-Human Trial of MIV-150 and Zinc Acetate Coformulated in a Carrageenan Gel: Safety, Pharmacokinetics, Acceptability, Adherence, and Pharmacodynamics. Journal of acquired immune deficiency syndromes (1999) 2016;73:489–96. doi: 10.1097/QAI.0000000000001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdool Karim SS, Abdool Karim Q, Kharsany AB, Baxter C, Grobler AC, Werner L, Kashuba A, Mansoor LE, Samsunder N, Mindel A, Gengiah TN, Group CT. Tenofovir Gel for the Prevention of Herpes Simplex Virus Type 2 Infection. The New England journal of medicine. 2015;373:530–9. doi: 10.1056/NEJMoa1410649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.