Fig. 3.
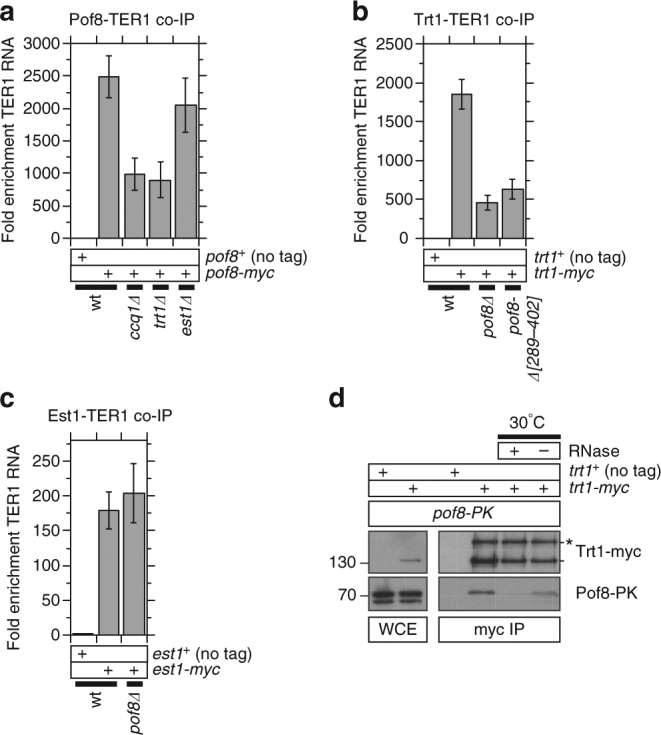
Pof8 binds strongly to telomerase RNA and facilitates interaction between Trt1 and telomerase RNA. a Binding of Pof8 to telomerase RNA TER1 was reduced in ccq1∆ and trt1∆, but not in est1∆ cells. Error bars correspond to SEM from at least 8 independent experiments. b TER1-Trt1 interaction was greatly diminished but not completely eliminated in pof8∆ or pof8-∆[289–402] cells. Error bars correspond to SEM from at least 4 independent experiments. c TER1-Est1 interaction was not affected by elimination of Pof8. Error bars correspond to SEM from at least 6 independent experiments. All TER1 co-IP plots show fold enrichment of TER1 RNA recovered after IP compared to no tag control strain. Raw data and statistical analysis are available in Supplementary Data 1. d Examination of Trt1-Pof8 interaction by co-IP. The Trt1-Pof8 interaction was nearly eliminated upon 10 min incubation at 30 oC in the presence of 1 mg/mL RNase A, while mostly retained in mock treatment without RNase A, suggesting that Trt1-Pof8 interaction is mediated by TER1 RNA. As previously reported70, anti-myc pull down of Trt1-myc showed enrichment of a higher molecular weight band marked with asterisk (*) not easily observed in whole cell extract (WCE). Molecular weight (kDa) of size markers are also indicated
