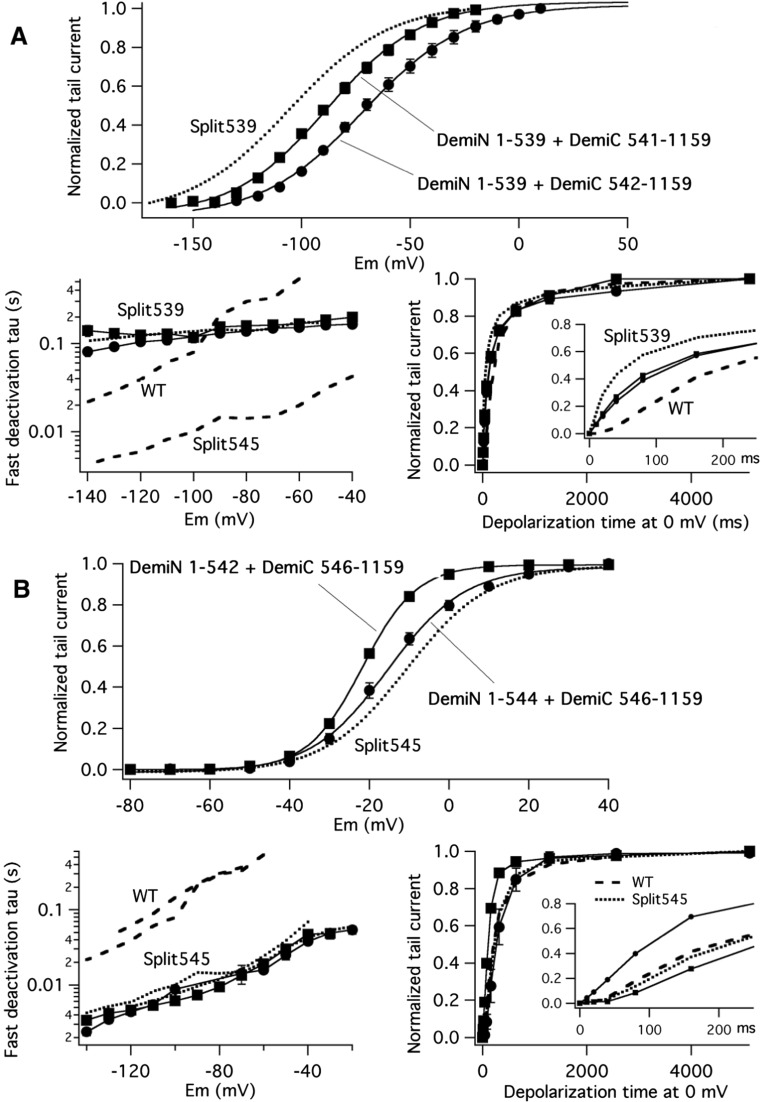
Fig. 4.
Functional properties of split channels carrying partial S4-S5 linker deletions around the disconnection point between the VSD and the PD. a Activation and deactivation gating kinetics of split 539 channels lacking Asp540 or Asp540 plus Arg541 residues. Activation voltage dependence (top). Fractional activation curves were obtained from oocytes co-expressing 1–539 plus 541–1159 or 1–539 plus 542–1159 demi-channel combinations (that will lack D540 or D540 plus R541 residues, respectively), using tail current data as detailed in Fig. 1 for split 539. Continuous lines are Boltzmann curves that best fitted the data as indicated in “Materials and methods” section. A Boltzmann curve from non-deleted split 539 channels as in Fig. 1d is also shown for comparison. Analogy of deactivation kinetics between L539 split channels with and without residues D540 and D540 plus R541 (bottom left). Deactivation time constants were quantified from double exponential fits to the tails as in Fig. 3 (see also “Materials and methods” section). Plots of deactivation time constants for the fast decaying component of the currents as a function of membrane potential are shown. Data from 1 to 539 plus 541–1159 (squares) and 1–539 plus 542–1159 (circles) demi-channel combinations appear superimposed to those from non-deleted split 539 channels (dotted line). Data from continuous wild-type and split 545 channels are also shown as dashed lines for comparison. Comparison of activation rates at 0 mV (bottom right). Averaged plots of normalized tail current magnitudes versus depolarization time at 0 mV are shown from 1 to 539 plus 541–1159 (squares) and 1–539 plus 542–1159 (circles) demi-channel combinations. An expansion of the initial 200-ms time course is shown in the inset. Data from continuous wild-type (dashed line) and non-deleted split 539 channels (dotted line) are also shown for comparison. b Activation and deactivation gating kinetics of split 545 channels lacking either Tyr545 or the Ser543 + Glu544 + Tyr545 segment. Analysis of activation voltage dependence (top), deactivation kinetics (bottom left) and the time course of current activation at 0 mV (bottom right) were performed as detailed in a. Data from oocytes expressing the 1–544 plus 546–1159 (lacking only the Y545 residue, circles) and the 1–542 plus 546–1159 (lacking the S543 + E544 + Y545 triplet, squares) demi-channel combinations are depicted. Due to the low level of expression obtained with the Y545-deleted construct, a high-K+ extracellular solution was used for the 1–542 plus 546–1159 combination recordings (see “Materials and methods” section). Data from continuous wild-type (dotted lines) and non-deleted split 545 channels (dashed lines) are also shown. In both cases, the traces indicating the slowest time constants correspond to data obtained in high-K+ extracellular solution