Abstract
Humans in strong social relationships are more likely to live longer because social relationships may buffer stressors and thus have protective effects. However, a shortcoming of human studies is that they often rely on self-reporting of these relationships. By contrast, observational studies of non-human animals permit detailed analyses of the specific nature of social relationships. Thus, discoveries that some social animals live longer and healthier lives if they are involved in social grooming, forage together or have more affiliative associates emphasizes the potential importance of social relationships on health and longevity. Previous studies have focused on the impact of social metrics on longevity in obligately social species. However, if sociality indeed has a key role in longevity, we might expect that affiliative relationships should also influence longevity in less social species. We focused on socially flexible yellow-bellied marmots (Marmota flaviventer) and asked whether female longevity covaries with the specific nature of social relationships. We quantified social relationships with social network statistics that were based on affiliative interactions, and then estimated the correlation between longevity and sociality using bivariate models. We found a significant negative phenotypic correlation between affiliative social relationship strength and longevity; marmots with greater degree, closeness and those with a greater negative average shortest path length died at younger ages. We conclude that sociality plays an important role in longevity, but how it does so may depend on whether a species is obligately or facultatively social.
Keywords: social networks, health, longevity, social behaviour, yellow-bellied marmots
1. Introduction
Affiliative social relationships affect longevity in human and non-human animals through a variety of mechanisms [1,2]. Strong affiliative social relationships enhance health and survival outcomes [3], but socially acquired pathogens [4] and social stressors [5] may reduce longevity. There are two main hypotheses that explain how strong affiliative relationships enhance longevity. The ‘buffering hypothesis’ suggests that the presence of an active social support system that provides emotional, informational or tangible aid to the focal individual, creates a buffer against acute or chronic stressors, such as illness and stressful life events [6]. The ‘main effects’ hypothesis proposes that social relationships provide direct emotional and behavioural aid that was not necessarily intended as support, but may encourage lifestyle changes such as healthy behaviours [6]. For example, the ‘main effects’ hypothesis is at work when people conform to social norms relevant to self-care due to the presence of these behaviours in their social network [6]. In addition, particularly in humans, complex social relationships that influence an individual's mental and physical health affect longevity through mechanisms such as social influence, sense of control, social control, role-based purpose and meaning, self-esteem, belonging and companionship, and perceived support availability [7]. A meta-analysis of 308 849 individuals across 148 studies revealed that humans with relatively strong social relationships increased their likelihood of survival by 50% [6]. Thus, improving human longevity requires an appropriate understanding of the specific ways in which social interactions influence longevity [5,8]. For instance, social interactions can be direct, and involve specific relationships with others or be more indirect and involve an individual's position in a more complex social network.
An important shortcoming of human studies is that they are often limited to self-reports and interviews. Self-reporting is conflated by biases, including social desirability bias, recall bias and confirmation bias [9]. These methods are also unable to identify the specific type of social relationship that may enhance health and longevity. By comparison, studies of individually marked animals permit researchers to directly observe and score social interactions, which permits detailed, objective analyses of the specific nature of social relationships.
Studies of longevity in non-humans have identified a number of ways in which sociality influences health and longevity. Using a ‘composite sociality index’ (CSI) to characterize affiliative relationships within dyads, it was shown that female chacma baboons (Papio hamadryas ursinas) which formed stronger and more stable social bonds with other females lived significantly longer than females that were in weaker and less stable relationships [10]. Similarly, female macaques with a higher degree in their social networks (i.e. they interacted more with conspecifics) or which had more relatives had higher survival [11]. In bighorn sheep (Ovis canadensis), which have fission–fusion social groups, female centrality (a measure of the degree to which individuals were well connected with others in their group) has a positive effect on lamb production and fitness [12]. Other affiliative social network metrics also capture the effects of direct and indirect social relationships on health and longevity. For example, adult longevity in rock hyraxes (Procavia capensis) was inversely correlated with the variation in a group's centrality [13]; hyraxes in groups with relatively more equal associations lived longer. Juvenile male bottlenose dolphins (Tursiops sp.) with higher eigenvector centrality, which takes into account indirect social relationships, had higher survival [14]. These results seem to suggest that in obligately social animals, longevity is enhanced in individuals with more associates, stronger bonds, and in those who were more connected with others in their group [15].
If sociality, or the two main hypotheses explaining it (‘buffering’ and ‘main effects’ hypotheses), has a key role in affecting longevity, we might expect that affiliative relationships would also influence longevity in less social or facultatively social species. By facultatively social, we mean that individuals have some degree of social flexibility and may be found in different size groups where they may or may not cooperate with others. We focused on facultatively social yellow-bellied marmots (Marmota flaviventer) and asked whether female longevity covaries with the strength of their social relationships. Marmots at our site have been studied since 1962 [16,17] and prior results have shown that they are often harem polygynous [17]. However, yellow-bellied marmots also live in a variety of group sizes, ranging from solitary females to females living with a single male, their young of the year and some yearlings, to females living in multi-female groups, with young and pre-dispersal yearlings from different females [17]. This social flexibility provides a unique opportunity to study the consequences of social variation [16]. They are also an excellent species in which to study the relationship between sociality and longevity because they are diurnal and can be easily located, trapped, marked and observed throughout their lives. Regular trapping and observations throughout the five to six month active season permits births and deaths of residents to be known with certainty. In addition, social relationships are not only heritable [18] but also have important ecological consequences [16,19,20].
We capitalized on marmot social flexibility to study the covariation between a suite of specific social network measures, which measure the quantity and strength of social relationships, and longevity. Both the ‘buffering’ and the ‘main effects’ hypotheses predict enhanced longevity for individuals with relatively more affiliative interactions. We, thus, hypothesize that marmots with higher social network trait values in affiliative interaction networks will live longer.
2. Material and methods
(a). Study site and subjects
Between 2002 and 2015, we studied yellow-bellied marmots located in the upper East River Valley in and around the Rocky Mountain Biological Laboratory, Gothic, Colorado (38°57′ N, 106°59′ W). We studied marmots along a 5 km section of a single valley that is subdivided into lower and higher elevation sections [17]. Marmots live in colonies and each colony contains one or more social groups, which are defined based on space-use overlap and burrow sharing [19,21]. Marmot colony sites vary in their suitability and some sites have been consistently occupied for the past 55 years, while others periodically go extinct (DT Blumstein 2017, unpublished observations) [22]. We examined 11 colonies that have been studied consistently since 2002, creating a dataset of 79 colony years (a colony studied for a year). During our study, these colonies ranged in size from 1 to 24 individuals (X ± s.d. = 6.4 ± 4.9).
Marmots were trapped on a bi-weekly basis every summer and individually marked for identification. All individuals trapped for the first time as juveniles or yearlings were thus of known age. Mortality was inferred for individuals not seen in consecutive years. Capture–mark–recapture analysis shows that greater than 98% of living individuals are captured annually [23], and thus longevity estimates for individuals of known age were accurate. Maximum female longevity at our study site is 16 years (DT Blumstein 2017, unpublished observations). Over-winter mortality and predation during the active season are the main sources of adult resident mortality [17] and when an otherwise healthy adult female suddenly disappears during the summer, we infer predation.
Stress hormone levels have previously been known to influence longevity via a variety of mechanisms [24]. We use faecal glucocorticoid metabolites as a measure of stress in marmots. Faecal samples were collected, when available, during capture events. Faecal samples were stored on ice and later frozen at −20°C. Faecal glucocorticoid metabolites were extracted and quantified at the end of each year using established protocols [25] and a validated assay [26].
(b). Social measures
Social interactions were recorded during near daily observations when it was not snowing or raining by observers quietly seated 20–150 m away using binoculars and 15–45× spotting scopes. Multiple observers recorded data in a given year and, before recording data, each observer was trained to identify subjects using their unique fur marks, and trained with our marmot social ethogram and videos of marmot interactions to consistently score social interactions. Individuals typically interact with members of the same group and observers recorded all social interactions between marmots that they could detect using all occurrence sampling [22]. Since social interactions are rare and obvious, they are missed using focal animal sampling; thus, all occurrence sampling is more appropriate in this system. In addition, because social interactions are rare, we included all interactions we could see. Thus, an individual could greet and then sit next to a conspecific and this would be scored as two unique social interactions. We also recognize that difficulties in detectability (because of vegetation and terrain) impact our ability to quantify all observations, but we have no way to correct for this. Observations occurred during hours of peak activity (07.00–10.00 in the morning and 16.00–19.00 in the afternoon from mid-April to mid-September [22]).
Since yellow-bellied marmots are matrilineal [17], we focused our study on female social relationships and removed both yearling males and adult males from all networks. There are no strong predictions about which specific measures of social relationship strength should be associated with longevity, so we created association matrices from a total of 5639 affiliative interactions among 247 female yearlings and female adults at 11 geographically distinct colony sites and created female only weighted social networks, which take into account the number of repetitive interactions that occurred between two individuals. We then calculated a variety of social network metrics that describe how connected individuals are, either directly or indirectly, with others in their social group. We focused on affiliative interactions [27,28] that included cohesive behaviours such as allogrooming, greeting, foraging together, playing together and sitting in close proximity [19].
To estimate an individual's sociality, we calculated 11 social network statistics (indegree, outdegree, incloseness, outcloseness, local clustering, global clustering, betweenness centrality, eigenvector centrality, average shortest path, instrength and outstrength) from the weighted social networks using the package iGraph v. 1.0.1 [29] in R v. 3.2.3 [30]. The final social networks we analysed were constructed from an average of 13.3 observations (s.d. = 18.2). Larger social groups were characterized by significantly more social observations (r = 0.92, p < 0.001).
Indegree describes the number of individuals a focal individual receives interactions from, whereas outdegree describes the number of individuals with whom a focal individual initiates interactions [31]. Closeness describes how influential a focal individual is by measuring how close (i.e. through direct or indirect relationships) they are to all other individuals in the network, defined as the reciprocal of the sum of the shortest path lengths between a focal individual and all other network individuals [30]. Incloseness and outcloseness describe received and initiated interactions, respectively. Clustering determines cliquishness of the network and is calculated by dividing the number of actual relationships formed between the focal individual and its neighbours by the total possible number of relationships that could be formed by the focal individual [32]. Local clustering describes the embeddedness of a focal individual and global clustering indicates the density of the network around a focal individual [31,33]. Betweenness centrality describes the proportion of shortest path lengths in the network between all other pairs of individuals connected to a focal individual [32]. Eigenvector centrality describes an individual's connectedness and takes into account the indirect relationships that occur between an individual's neighbours [34]. Average shortest path describes the efficiency of a network in transferring information and is calculated from the average number of individuals that a focal individual must go through in order to contact another member of its social network [34]. Strength describes the sum of the weights (frequency) of interactions between a focal individual and their adjacent neighbours [31,33]. Instrength and outstrength describe received and initiated interactions, respectively.
For all estimated social network attributes, except average shortest path, a larger value is interpreted as being more social. Conversely, for average shortest path, a larger value is interpreted as being less social. Thus, to facilitate the interpretation, we used the opposite of average shortest path which we refer to as ‘negative average shortest path’. Thus, following our hypothesis, we expect positive correlations between all social network traits and longevity.
(c). Statistical analyses
To test the influence of sociality on longevity, we fitted bivariate models of weighted social network metrics and longevity. To avoid selective disappearance biases, we restricted our analysis to extinct cohorts only. The final dataset included 345 estimates for each of the 11 affiliative social network attributes for 66 unique individuals (some traits could not be calculated for each individual). Each bivariate model fitted a social network trait and log-transformed longevity as dependent variables. To facilitate model convergence and allow for comparison across traits, all variables were scaled with a mean of zero and a variance of one. For social network traits, valley, age and log-transformed faecal glucocorticoid metabolite level were fitted as fixed effects to correct for environmental, ageing and stress effects on sociality. Year was fitted as a random effect to account for annual variation in population structure. For longevity, valley was included as a fixed effect, and year of birth was fitted as a random effect to account for cohort effects. We fitted individual identity as a random effect for both traits. Since each individual has only one observation for longevity, we fixed the longevity residual variance and the residual covariance at 0, allowing us to estimate the covariation between longevity and social network traits at the individual level.
Models were fitted using a Bayesian approach using MCMCglmm [35] in R v. 3.2.3 [30]. We used flat priors at the correlation level for individual identity effect (i.e. parameter expanded prior: V = diag(2), ν = 3, α.μ = rep(0,2), α.V = diag(25^2,2)). Priors for cohort and year random effects were uninformative (V = 1 and ν = 0.002). The prior for the residual variance was uninformative for social traits and fixed at 0 for longevity (V = diag(1, 0.00002), ν = 1.002, fix = 2). Each bivariate model was run for 2 300 000 iterations with a thinning of 2000 and a burning period of 300 000 iterations, which, for all parameters, produced autocorrelation coefficients less than 0.1 and effective samples size between 910 and 1000.
3. Results
Some, but not all, of our fixed effects were significant; we focus first on network traits, and then on longevity. No variation in social traits was explained by FGM (table 1), a finding that suggests no relation between stress and sociality. Age was significantly related only with eigenvector centrality (table 1). Eigenvector centrality decreased with age suggesting that older females were less social; a finding previously reported [36]. Valley was significant only for global clustering, with individuals up-valley having higher clustering (table 1). There was no effect of valley on longevity (electronic supplementary material, table S3).
Table 1.
Fixed effects fitted on social network traits within bivariate models of social network traits and longevity. Down valley was used as a reference level. We report estimates with lower and upper 95% credible intervals between parentheses. Estimates in italics were significantly different from zero.
| social network trait | intercept | faecal glucocorticoid metabolites | age | position in valley (up) |
|---|---|---|---|---|
| negative average shortest path | 0.425 (−0.064/0.929) | 0.100 (−0.021/0.217) | 0.028 (−0.033/0.089) | −0.376 (−0.785/0.055) |
| betweenness | 0.524 (−0.023/1.071) | −0.135 (−0.309/0.045) | −0.005 (−0.081/0.074) | −0.409 (−0.970/0.158) |
| eigenvector centrality | 0.564 (0.022/1.0787) | 0.066 (−0.113/0.247) | −0.114 (−0.186/−0.044) | 0.058 (−0.401/0.548) |
| global clustering | −0.352 (−0.805/0.0971) | −0.141 (−0.331/0.049) | 0.0211 (−0.048/0.090) | 0.471 (0.015/0.906) |
| incloseness | 0.855 (0.201/1.484) | −0.013 (−0.140/0.116) | −0.025 (−0.097/0.048) | −0.336 (−0.828/0.150) |
| indegree | 0.873 (0.293/1.484) | −0.067 (−0.243/0.106) | −0.071 (−0.139/0.001) | −0.262 (−0.692/0.199) |
| instrength | 0.429 (−0.164/1.010) | −0.043 (−0.239/0.140) | −0.080 (−0.140/−0.020) | −0.126 (−0.484/0.230) |
| local clustering | −0.286 (−1.179/0.651) | −0.124 (−0.306/0.050) | 0.135 (0.051/0.225) | 0.261 (−0.304/0.864) |
| outcloseness | 0.594 (−0.037/1.185) | −0.005 (−0.131/0.110) | 0.019 (−0.053/0.091) | −0.198 (−0.687/0.289) |
| outdegree | 0.414 (−0.0860/0.903) | −0.048 (−0.212/0.111) | 0.005 (−0.070/0.071) | −0.151 (−0.648/0.354) |
| outstrength | 0.507 (−0.004/1.071) | −0.071 (−0.251/0.115) | −0.062 (−0.138/0.018) | −0.341 (−0.829/0.168) |
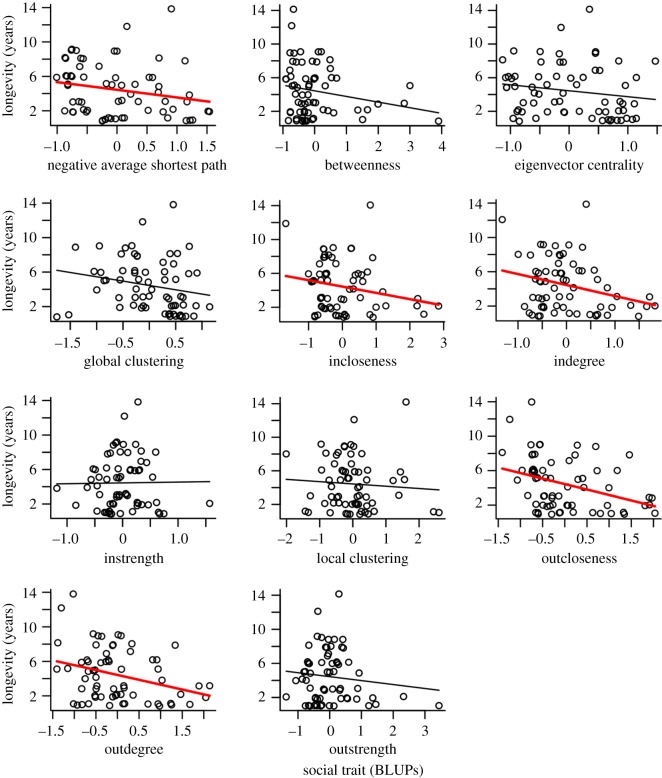
All social network traits were repeatable with a non-zero between-individual variance (table 2), and between-individual variance in longevity was similar across all models (table 2). Out of 11 correlations between social traits and longevity, nine were negative, five of them were significantly so and one more (betweenness) cannot be said to be significantly different from zero, but is still negative because 96.5% of its posterior distribution was negative (table 2 and figure 1). The two positive correlations were small and not significantly different from zero (table 2). This was in stark contrast to our expectations that social attributes would generally be positively correlated with longevity. Marmots who were closer to others within their network, defined by greater indegree and outdegree, incloseness and outcloseness, and negative average shortest path length, lived shorter lives (table 2, figure 1). Results were similar (betweenness became significantly negative) when analyses were conducted on un-weighted social network measures (electronic supplementary material, table S1).
Table 2.
Results of bivariate models illustrating the variance, covariance and correlation at the individual level between longevity and weighted affiliative social traits. We report the estimates with lower and upper 95% credible intervals between parentheses. Estimates in italic were significantly different from zero.
| social network trait | VSNT | Vlongevity | correlation |
|---|---|---|---|
| negative average shortest path | 0.620 (0.385/0.882) | 0.356 (0.222/0.507) | −0.417 (−0.636/−0.103) |
| betweenness | 1.106 (0.662/1.607) | 0.358 (0.226/0.509) | −0.280 (−0.525/0.002) |
| eigenvector centrality | 0.732 (0.431/1.091) | 0.372 (0.231/0.528) | −0.277 (−0.545/0.016) |
| global clustering | 0.584 (0.314/0.901) | 0.360 (0.219/0.513) | −0.117 (−0.472/0.153) |
| incloseness | 0.887 (0.550/1.257) | 0.346 (0.215/0.492) | −0.493 (−0.667/−0.183) |
| indegree | 0.622 (0.341/0.955) | 0.362 (0.228/0.509) | −0.393 (−0.650/−0.138) |
| instrength | 0.308 (0.136/0.499) | 0.361 (0.230/0.517) | 0.120 (−0.220/0.448) |
| local clustering | 0.976 (0.543/1.482) | 0.369 (0.230/0.525) | −0.0539 (−0.364/0.309) |
| outcloseness | 0.864 (0.555/1.211) | 0.346 (0.214/0.491) | −0.596 (−0.778/−0.378) |
| outdegree | 0.875 (0.536/1.256) | 0.351 (0.219/0.501) | −0.462 (−0.686/−0.195) |
| outstrength | 0.795 (0.431/1.195) | 0.365 (0.229/0.516) | 0.0165 (−0.247/0.358) |
Figure 1.
Relationship between social network traits and longevity in yellow-bellied marmots. Best linear unbiased predictors (BLUPs) were used for social network traits for illustrative purposes. Each point represents an individual. Lines represent the relation between social trait and longevity, estimated as the linear regression between social trait BLUP and longevity. Bold red lines are significantly different from zero according to table 1. (Online version in colour.)
4. Discussion
Previous studies in humans [5] and other obligately social mammals (e.g. [11,12]) identified significant positive correlations between individuals being in strong, affiliative social relationships and longevity. In yellow-bellied marmots, a facultatively social mammal, this pattern does not hold. We used formal social network measures of relationship strength, a relatively large sample size of long-lived free-living mammals, and the appropriate bivariate model that permitted us to isolate and estimate the covariance between sociality and longevity. We found that five of the 11 affiliative social network traits we measured were significantly associated with reduced lifespans. Stated succinctly, more social animals lived shorter lives. These results are inconsistent with what has been previously reported in humans and other species (see Introduction) and do not support either the buffering or main effects hypotheses, which state that increased sociality should correlate with increasing longevity. Thus, these marmot results suggest that, in some species, strong social relationships need not be beneficial and may even be costly. In retrospect, the results may have been anticipated by a recent finding that yellow-bellied marmots with stronger affiliative relationships were more likely to die over-winter [20]. While that result focused solely on over-winter survival, the current study focused on overall longevity—which is influenced by both summer survival and over-winter survival. Thus, this study was more comprehensive and explored the influence of more social network traits.
However, these somewhat paradoxical results are consistent with a suite of other findings from our population of yellow-bellied marmots. Female marmots in stronger affiliative relationships have reduced annual reproductive success [19], and marmots with stronger affiliative relationships during the summer are less likely to survive the following winter [20]. Older females become less social with age [36], and homophily rules that describe interactions based on age, sex and relatedness, seem to break down at larger group sizes [37]. Finally, there is significant heritable variation in the propensity to tolerate agonistic behaviour, but no significant heritable variation in the propensity to engage in affiliative behaviour [18]. Thus, while marmots are social, they are not necessarily cooperative [16] and they may not necessarily benefit from strong social relationships.
When population size increases and animals fail to disperse, marmots have the potential to interact with more individuals. However, individuals in larger groups do not necessarily benefit from increased social connectivity. In situations where dispersing animals leave a site with a relatively high probability of survival to go off to an uncertain fate, increased social connectivity may be adaptive for individuals that are otherwise likely to disperse because it strengthens relationships and reduces the likelihood of dispersal. Nevertheless, for established residents, increased social connectivity may be costly. This should be expected in situations where insider–outsider conflicts exist [38,39]. In such situations, residents pay a cost when outsiders join groups, while outsiders join groups because they obtain greater fitness living socially compared to that when living alone.
Given our findings that social relationships are significantly negatively correlated with longevity in a facultatively social species, variation in social relationships may have profoundly different effects on population demography in highly social (e.g. obligately social species) and less social species (e.g. facultatively social and asocial species). Our results also suggest that both direct affiliative social relationships (degree and strength) as well as less direct affiliative relationships (betweeness centrality) may affect longevity. Additionally, the significance of both indegree and outdegree suggests that it is not only affiliative actions that individuals initiated that negatively influence longevity, but also affiliative actions that they received and have little control over. Our findings prompt future questions about why affiliative interactions in particular have negative effects on individual survival.
Our results are inconsistent with both the buffering and main effects hypotheses; affiliative social interactions decreased longevity, suggesting that affiliative interaction does not act as a buffer to stress in the same ways that it does in other animals. We, nevertheless, illustrated the seminal importance of sociality effects on longevity. Additional studies on other facultatively social species may reveal whether this negative phenotypic correlation between sociality and longevity is typical of facultative sociality and would answer the question of whether being ‘too social’ may be costly in some species. Additionally, studies of systems characterized by insider–outsider dynamics that are created when animals disperse and must try to settle in existing groups may also be revealing. Focusing further studies on variation in the benefits of sociality for individuals within a species, including humans, may produce more evidence that all individuals do not benefit equally from maintaining strong social relationships; a provocative suggestion that requires further study.
Supplementary Material
Ethics
Marmots were studied under annual permits issued by the Colorado Division of Wildlife (TR-917). All procedures were approved under research protocol ARC 2001-191-01 by the University of California Los Angeles Animal Care Committee on 13 May 2002, and renewed annually.
Data accessibility
Data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.h8n7p [40].
Authors' contributions
D.T.B. conceived idea and designed analyses with J.G.A.M., D.M.W. and A.N.L. D.T.B., D.M.W., S.K. and J.G.A.M. collected the data. J.G.A.M., D.M.W. and A.N.L. analysed the data. All authors interpreted the data and contributed to manuscript writing.
Competing interests
We declare we have no competing interests.
Funding
D.T.B. was supported by the National Geographic Society, UCLA (Faculty Senate and the Division of Life Sciences), a Rocky Mountain Biological Laboratory research fellowship and by the NSF (IDBR-0754247, and DEB-1119660 and 1557130 to D.T.B., as well as DBI-0242960, 0731346 and 1226713 to the Rocky Mountain Biological Laboratory).
References
- 1.Carey JR. 2003. Longevity: the biology and demography of life span. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Hoenig JM. 2005. Empirical use of longevity data to estimate mortality rates. SEDAR33- RD17. SEDAR, North Charleston, SC. 8.
- 3.Umberson D, Montez JK. 2011. Social relationships and health: a flashpoint for health policy. J. Health. Soc. Behav. 51, S54–S66. ( 10.1177/0022146510383501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cusumano-Towner M, Li DY, Tuo S, Krishnan G, Maslove DM. 2013. A social network of hospital acquired infection built from electronic medical record data. J. Am. Med. Inform. Assoc. 20, 427–434. ( 10.1136/amiajnl-2012-001401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. 2016. Social relationships and physiological determinants of longevity across the human life span. Proc. Natl Acad. Sci. USA 113, 578–583. ( 10.1073/pnas.1511085112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt-Lunstad K, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic. PLoS Med. 7, e1000316 ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoits PA. 2011. Mechanisms linking social ties and support to physical and mental health. J. Health Soc. Behav. 52, 145–161. ( 10.1177/0022146510395592) [DOI] [PubMed] [Google Scholar]
- 8.McCowan B, Beisner B, Bliss-Moreau E, Vandeleest J, Jin J, Hannibal D, Hsieh F. 2016. Connections matter: social networks and lifespan health in primate translational models. Front. Psychol. 7, 433 ( 10.3389/fpsyg.2016.00433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Althubaiti A. 2016. Information bias in health research: definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 9, 211–217. ( 10.2147/JMDH.S104807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JB, Beehner JC, Bergmann TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. ( 10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 11.Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Family networks size and survival across the lifespan of female macaques. Proc. R. Soc. B 284: 20170701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Wal E, Festa-Bianchet M, Réale D, Coltman DW, Pelletier F. 2015. Sex-based differences in the adaptive value of social behavior contrasted against morphology and environment. Ecology 96, 631–641. ( 10.1890/14-1320.1) [DOI] [PubMed] [Google Scholar]
- 13.Barocas A, Ilany A, Koren L, Kam M, Geffen E. 2011. Variance in centrality within rock hyrax social networks predicts adult longevity. PLoS ONE 6, e22375 ( 10.1371/journal.pone.0022375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanton MA, Mann J. 2012. Early social networks predict survival in wild bottlenose dolphins. PLoS ONE 7, e47508 ( 10.1371/journal.pone.0047508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappeler PM, Cremer S, Nunn CL. 2015. Sociality and health: impacts of sociality on disease susceptibility and transmission in animal and human societies. Phil. Trans. R. Soc. B 370, 20140116 ( 10.1098/rstb.2014.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumstein DT. 2013. Yellow-bellied marmots: insights from an emergent view of sociality. Phil. Trans. R. Soc. B 368, 20120349 ( 10.1098/rstb.2012.0349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armitage KB. 2014. Marmot biology: sociality, individual fitness, and population dynamics. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Lea AJ, Blumstein DT, Wey TW, Martin JGA. 2010. Heritable victimization and the benefits of agonistic relationships. Proc. Natl Acad. Sci. USA 107, 21 587–21 592. ( 10.1073/pnas.1009882107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wey TW, Blumstein DT. 2012. Social attributes and associated performance measures in marmots: bigger male bullies and weakly affiliating females have higher annual reproductive success. Behav. Ecol. Sociobiol. 66, 1075–1085. ( 10.1007/s00265-012-1358-8) [DOI] [Google Scholar]
- 20.Yang WJ, Maldonado-Chaparro A, Blumstein DT. 2017. A cost of being amicable in a hibernating marmot. Behav. Ecol. 28, 11–19. ( 10.1093/beheco/arw125) [DOI] [Google Scholar]
- 21.Blumstein DT, Wey TW, Tang K. 2009. A test of the social cohesion hypothesis: interactive female marmots remain at home. Proc. R. Soc. B 276, 3007–3012. ( 10.1098/rspb.2009.0703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumstein DT, Ozgul A, Yovovitch V, Van Vuren DH, Armitage KB. 2006. Effect of predation risk on the presence and persistence of yellow-bellied marmot (Marmota flaviventris) colonies. J. Zool. Lond. 270, 132–138. ( 10.1111/j.1469-7998.2006.00098.x) [DOI] [Google Scholar]
- 23.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson ST, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature. 466, 482–485. ( 10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavretsky H, Newhouse PA. 2012. Stress, inflammation and aging. Am. J. Geriatr. Psychiatry 20, 729–733. ( 10.1097/JGP.0b013e31826573cf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumstein DT, Patton ML, Saltzman W. 2006. Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol. Lett. 2, 29–32. ( 10.1098/rsbl.2005.0405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JE, Monclús R, Wantuck D, Florant GL, Blumstein DT. 2012. Fecal glucocorticoid metabolites in wild yellow-bellied marmots: experimental validation, individual differences and ecological correlates. Gen. Comp. Endocrinol. 178, 417–426. ( 10.1016/j.ygcen.2012.06.015) [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Wey TW, Blumstein DT. 2011. Correlates and consequences of dominance in a social rodent. Ethology 117, 573–585. ( 10.1111/j.1439-0310.2011.01909.x) [DOI] [Google Scholar]
- 28.Fuong H, Maldonado-Chaparro A, Blumstein DT. 2015. Are social attributes associated with alarm calling propensity. Behav. Ecol. 26, 587–592. ( 10.1093/beheco/aru235) [DOI] [Google Scholar]
- 29.Csardi G, Nepusz T. 2006. The igraph software package for complex network research. Int. J. Complex Syst. 1695 See http://igraph.org. [Google Scholar]
- 30.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 31.Wasserman S, Faust K. 1994. Social network analysis: methods and application. New York, NY: Cambridge University Press. [Google Scholar]
- 32.Wey T, Blumstein DT, Shen W, Jordan F. 2008. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 75, 333–344. ( 10.1016/j.anbehav.2007.06.020) [DOI] [Google Scholar]
- 33.Barrat A, Barthelemy M, Pastor-Satorras R, Vespignani A. 2004. The architecture of complex weighted networks. Proc. Natl Acad. Sci. USA 101, 3747–3752. ( 10.1073/pnas.0400087101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman MEJ. 2010. Networks: an introduction. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Hadfield JD. 2010. MCMC methods for multi–response generalized linear mixed models: the MCMCglmm R package. J. Stat. Soft. 33, 1–22. ( 10.18637/jss.v033.i02) [DOI] [Google Scholar]
- 36.Wey TW, Blumstein DT. 2010. Social cohesion in yellow-bellied marmots is established through age and kin structuring. Anim. Behav. 79, 1343–1352. ( 10.1016/j.anbehav.2010.03.008) [DOI] [Google Scholar]
- 37.Yang WJ, Maldonado-Chaparro AA, Blumstein DT. Submitted. Identifying the behavioral rules underlying social network structure in yellow-bellied marmots (Marmota flaviventer). Ecol. Model. [Google Scholar]
- 38.Higashi M, Yamamura N. 1994. Resolution of evolutionary conflict: a general theory and its applications. Pop. Ecol. Rev. 36, 15–22. ( 10.1007/BF02515080) [DOI] [Google Scholar]
- 39.Giraldeau L, Caraco T. 2000. Social foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- 40.Blumstein D, Williams D, Lim A, Kroeger S, Martin J. 2017. Data from: Strong social relationships are associated with decreased longevity in a facultatively social mammal. Dryad Digital Repository. ( 10.5061/dryad.h8n7p). [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Blumstein D, Williams D, Lim A, Kroeger S, Martin J. 2017. Data from: Strong social relationships are associated with decreased longevity in a facultatively social mammal. Dryad Digital Repository. ( 10.5061/dryad.h8n7p). [DOI]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.h8n7p [40].