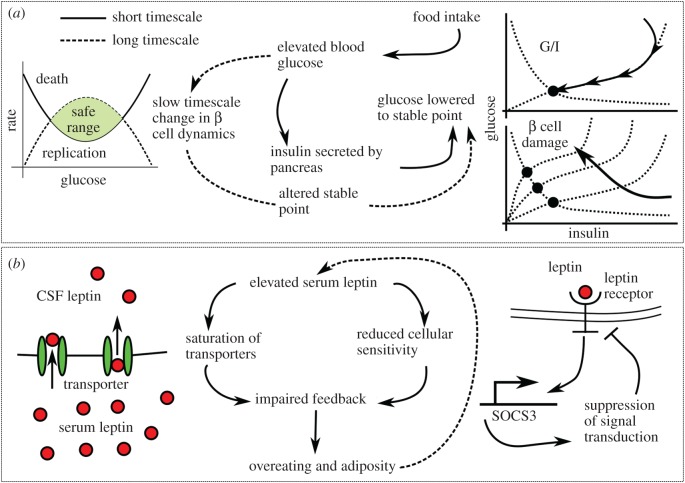
Figure 2.
(a) Dynamical systems models of glucostasis illustrate the importance of considering both short- and long-term behaviour. The schematic on the left illustrates the interplay between short-term glucostasis due to the action of insulin and the long-term effect of elevated glucose on the β cells in the pancreas. Initially, the glucose/insulin system is at a fixed point: glucose and insulin concentrations are stable. After receiving a glucose spike, for instance following a meal, the system evolves towards a new set point at a higher glucose concentration. Glucose levels above a certain level lead to pancreatic β cell death (shaded region) and the amount of time the system spends in this region, as well as the amount glucose levels exceed the threshold, determine the level of β cell damage. This damage reduces insulin secretion, which in turn moves the fixed point to a new value. The degree to which this movement occurs in a single cycle has been exaggerated to increase the clarity of the figure. (b) A similar model of leptin resistance, in which leptin receptor density depends nonlinearly on leptin concentration, also shows a rich phenomenology. As the effect of leptin concentration on food intake and the rate at which excess leptin concentration causes receptor desensitization are varied (as can happen when exposed to more palatable food and during ageing, respectively), the steady state of the system can vary sharply. A mouse with initial low body fat will return to a healthy steady state, whereas an obese one will return to obesity following a perturbation.