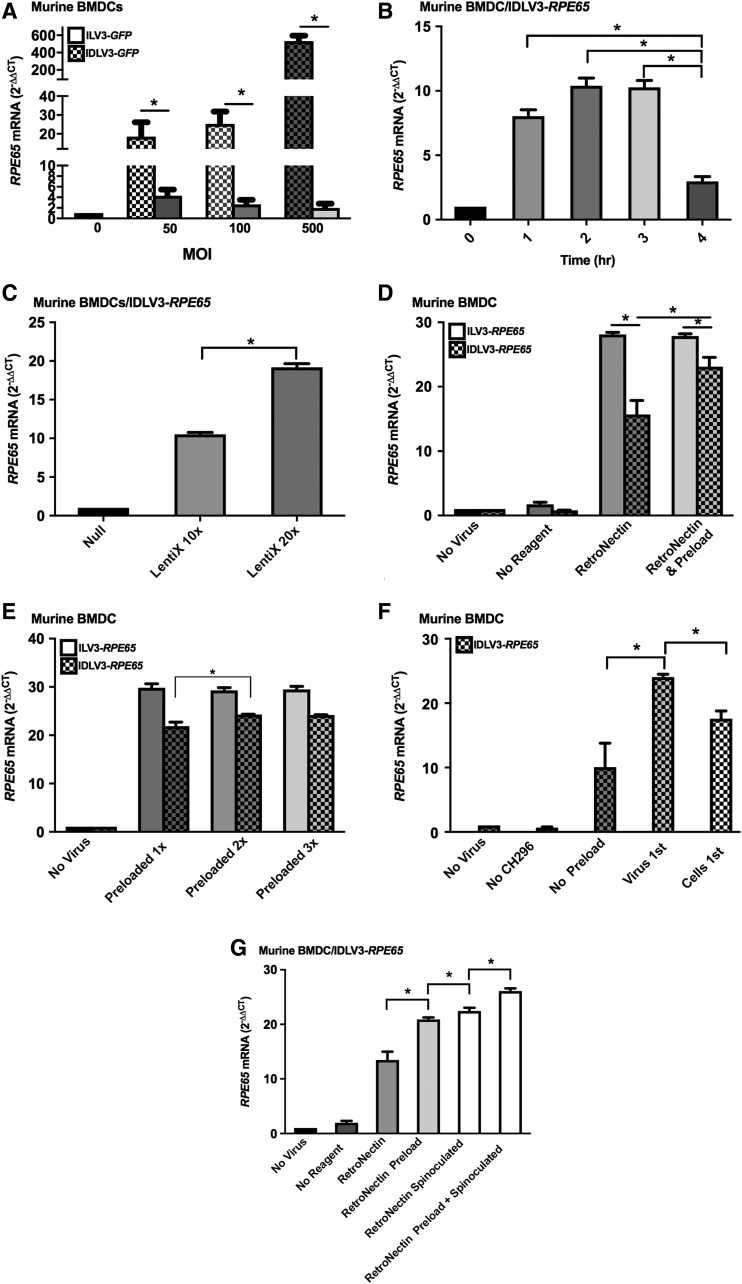
Figure 3.
Enhancing the infection of BMDCs with IDLV3-RPE65. Murine (Lin–/Sca1+) or human (CD34+) BMDCs were infected ILV3-RPE65 or IDLV-RPE65 (at a MOI of 50 (A, C–G), 100 (A), or 500 (A), or with 20 μL concentrated viral supernatant (B). RetroNectin at 2 μg/cm2 was used for all transductions, and was either used by itself (A–C) or with preloading of vectors (D–G) or cells (F). Cells were infected for 12 h and harvested for analysis at 16 h unless spinoculated (G), where cells were infected for 2 h at 150 g and harvested for analysis at 4 h. (A) Increasing the MOI of ILV3-RPE65 increased RPE65 mRNA levels from ∼27-fold over control (p < 0.05) at MOI 50 to ∼68-fold over control (p < 0.05) at MOI 100 and ∼150-fold over control (p < 0.05) with MOI 500. Increasing the MOI of IDLV3-RPE65 did had no significant effect on expression, with RPE65 levels remaining at around sevenfold over control (p < 0.05). (B) Centrifugation of IDLV3-RPE65 supernatant for 2 h increased recovery of virions, with a ∼10-fold increase in infection of murine BMDCs with concentrated supernatant at the same volume in comparison to eightfold observed at 1 h. Centrifugation for 3 h did not enhance infection efficiency, and centrifuging for 4 h reduced the viability of virions, with RPE65 expression only threefold over control (p < 0.05). (C) Concentrating viral supernatant 20 × enhanced the infection of IDLV3-RPE65 in comparison with concentrating 10 × and infecting at the same MOI, with a 19-fold (p < 0.05) increase in RPE65 mRNA observed at a MOI of 50 with the 10 × concentrated vector and 10-fold (p < 0.05) with the 20 × concentrated vector at the same MOI. (D) Preloading ILV3-RPE65 on RetroNectin for 30 min prior to adding murine BMDCs did not enhance infection, with a 28-fold increase (p < 0.05) observed with or without preloading. In contrast, with IDLV3-RPE65, preloading increased RPE65 expression from 15-fold over control (p < 0.05) to 23-fold over control (p < 0.05). (E) Preloading ILV3-RPE65 on RetroNectin up to three times did not result in an increase in human RPE65 expression. Preloading IDLV3-RPE65 twice increased infection, with RPE65 mRNA levels increasing from 21- to 24-fold over control (p < 0.05). No increase was observed with three preloads. (F) Murine BMDCs transduced with IDLV-RPE65 expressed RPE65 at the highest level when viral particles were preloaded onto RetroNectin, with a 24-fold increase (p < 0.05) in expression versus 10-fold with no preload (p < 0.05). Preloading the cells instead of the vector yielded an increase in RPE65 expression, with a 17-fold increase versus control (p < 0.05). However, this was lower than observed when viral particles were preloaded, indicating no advantage in preloading cells (G) IDLV-RPE65 infects murine BMDCs at the highest efficiency when the vector is preloaded onto RetroNectin followed by infection by spinoculation, with an 26-fold increase (p < 0.05) in expression over control in comparison to a 20-fold increase with preload alone (p < 0.05), and a 22-fold increase with spinoculation alone (p < 0.05). (*p < 0.05 [SEM]; n = 3). All statistical significance was calculated using one-way ANOVA followed by Tukey's multiple comparisons test.