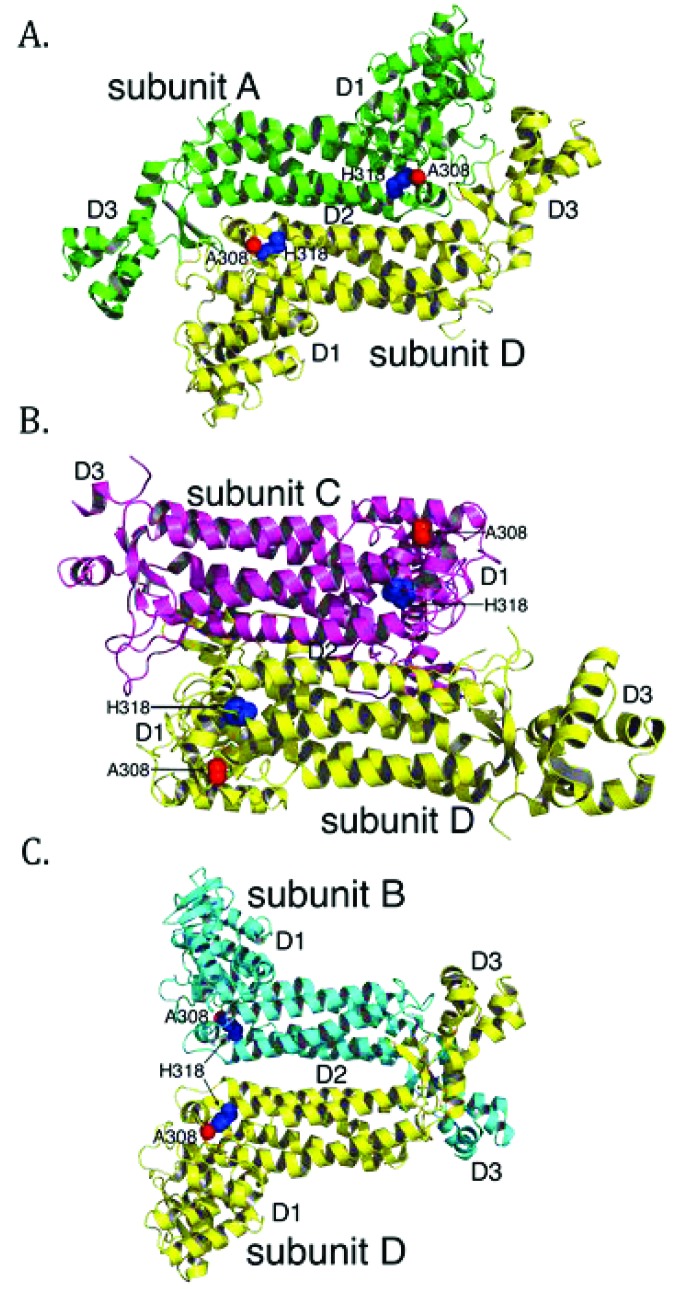
Fig. (3).
The positions of A308 and H318 differ within the three symmetry related dimer combinations of the human fumarase structure (PDB ID: 3E04 [40];). Three dimer interfaces derive from the fumarase homotetramer: (A) subunit A-subunit D (A-D), (B) subunit C-subunit D (C-D), and (C) subunit B-subunit D (B-D). The average calculated surface area across the three dimers is 3,000 (A-D), 2,300 (C-D) and 1,700 Å2 (B-D), respectively. Structurally, the intersubunit positions of A308 and H318 are nearest within the A-D dimer. The A-D dimer forms via interactions between residues donated by α-helices 12 and 13 of neighboring subunits. At the C-D dimer interface the positions of A308 and H318 are spatially removed. The B-D dimer shares the least amount of interacting surface area and the positions of A308 and H318 (within each monomer) are spatially distant. Monomers have been colored as depicted in Fig. 1B.