FIG 2 .
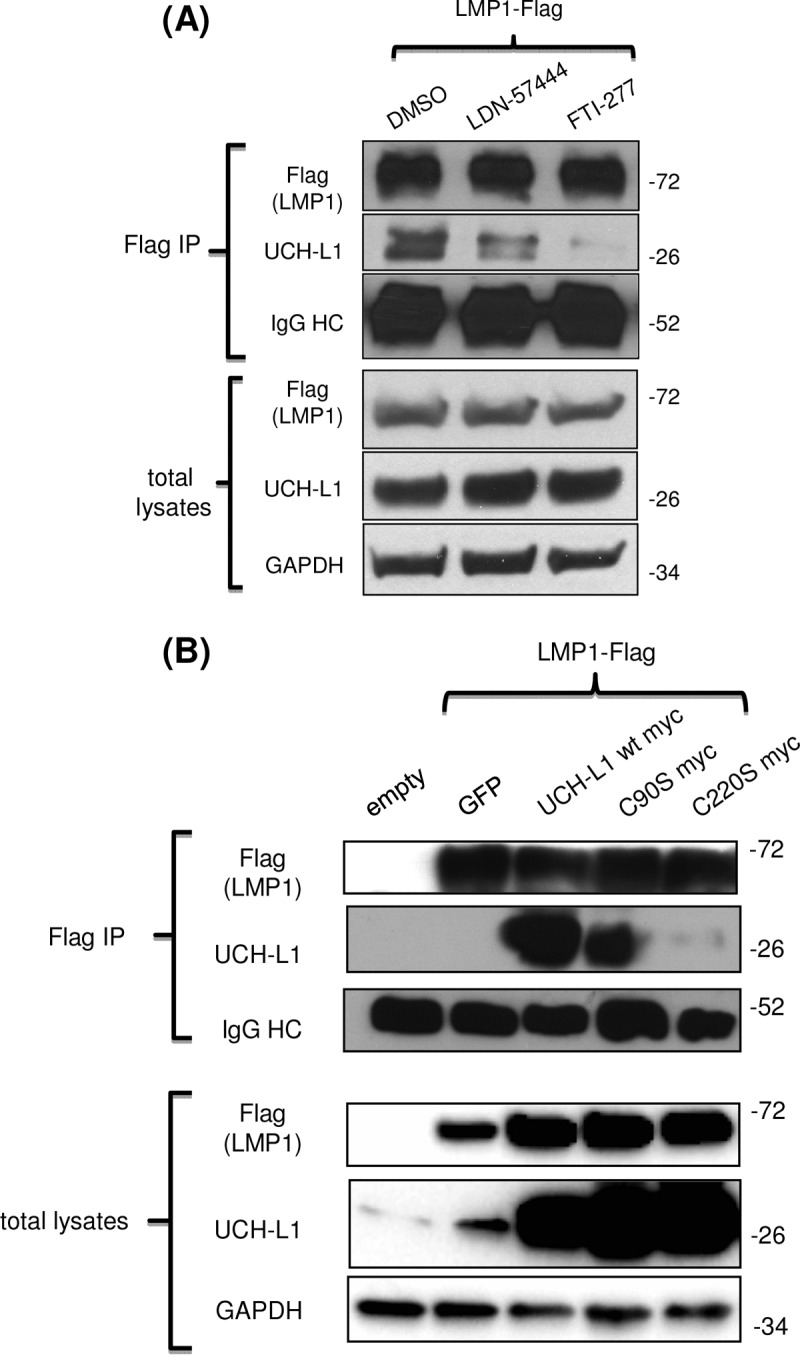
C-terminal farnesylation of UCH-L1 is required for its association with the EBV primary oncogene LMP1. (A) Inhibition of cellular farnesylation reduces LMP1 association with UCH-L1. 293 cells were transfected with empty vector, GFP (green fluorescent protein), LMP1-Flag and UCH-L1 wild-type expression vectors and treated with DMSO and either UCH-L1 DUB activity inhibitor LDN-57444 or farnesyltransferase inhibitor FTI-277 (5 μM each). At 48 h after transfection, LMP1 was immunoprecipitated with anti-Flag-agarose beads. Band intensity was quantified by the use of ImageJ (http://rsbweb.nih.gov/ij/) software. The result shows less UCH-L1 in the LMP1 complexes under conditions of treatment with FTI-277 than was seen with the DMSO control or LDN-57444 treatment. (B) Inhibition of UCH-L1-specific farnesylation inhibits LMP1/UCH-L1 complex formation. 293 cells were transfected with LMP1-Flag and the UCH-L1 wild type or one of two UCH-L1 mutants: an enzymatically inactive mutant (C90S mutant) or UCH-L1 with a mutated farnesylation site (C220S mutant). Cells were harvested 48 h posttransfection for LMP1 complex formation analysis. After IP with anti-Flag-agarose beads, Western blot analysis was performed with the indicated antibodies. The results revealed less UCH-L1 C220S mutant than wild type or C90S mutant in complex with LMP1.
