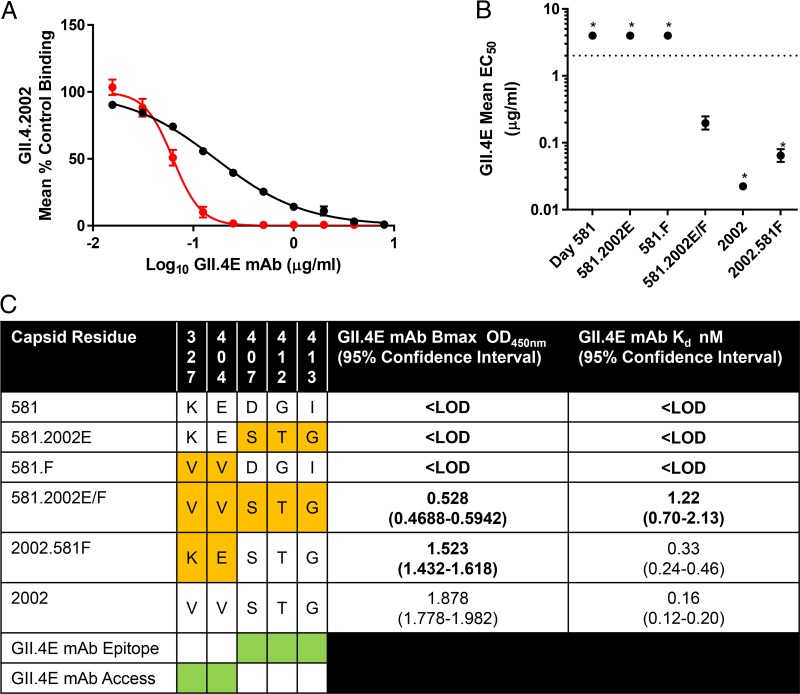
FIG 6 .
GII.4E MAb binding is regulated by global particle conformation and surrounding local topology. (A) GII.4.2002 VLP binding to ligand was blocked by GII.4E at either room temperature (black) or 37°C (red), and mean percent control binding compared to no antibody was fit by sigmoidal dose-response curve analysis, with the Hill slopes and EC50s calculated. Incubation at 37°C resulted in a steeper curve and lower EC50 titer, indicating that antibody access to epitope E is dependent upon particle conformation, as described for epitopes F and G. (B) Exchange of both epitope E and F residues into the 581 backbone (581.2002E/F) improved binding of GII.4E MAb compared to exchanging only either epitope E (581.2002E) or epitope F (581.F) residues. Values that are significantly different (P < 0.05) from the value for 581.2002E/F are indicated by an asterisk. (C) To distinguish the effects of epitope E and F residues on GII.4E binding, chimeric VLPs (orange) were tested for GII.4E binding by EIA, and Bmax and Kd values were calculated. Exchange of day 581 epitope F residues into the 2002 backbone (2002.581F) did not change the Kd for GII.4E but did decrease the Bmax, indicating that epitope F residues mediate GII.4E epitope access. In support of this hypothesis, exchange of both epitope E and F residues from the 2002 backbone into the 581 backbone (581.2002E/F) was necessary to restore GII.4E binding. Bold values are significantly different (P < 0.05) from GII.4.2002. Green shading indicates residues involved in GII.4E epitope binding and access to the epitope. LOD, less than the limit of detection.