Fig. 4.
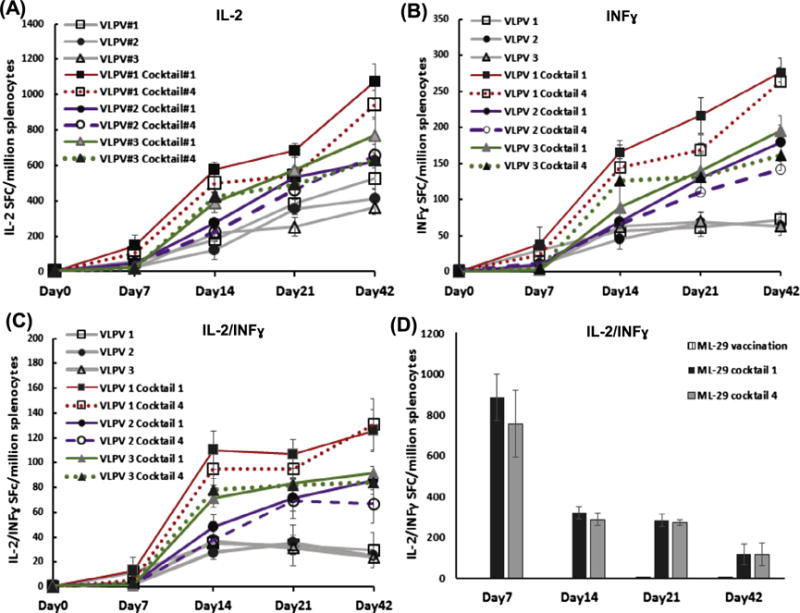
Cross-reactive T cell responses in VLPV-infected mice. (A)-(C), vaccination with VLPV1, VLPV2 and VLPV3 (blended formulation, VLPV1&VLPV2), respectively. VLPV1 antigen formulation is LNΔGPfib/LJGPwt; VLPV2 is LJΔGPfib/LNGPwt, where LJ and LN, LASV-Josiah and LASV-Nigeria from distantly-related phylogenetic groups IV and I, respectively. GPwt and ΔGpfib, wild-type and genetically modified LASV GP, respectively (see Fig. 1). (D), vaccination with ML29. Splenocytes from vaccinated and challenged mice were collected at indicated time points, stimulated with GP peptide cocktails I (LASV/LP/NIG) or IV (LASV/JOS/SL) and cells secreting individual cytokines, IL-2 (panel A), IFN-γ (panel B), or both, IFN-γ/IL-2 (panels C and D), were counted as described in Methods.
