Abstract
Objectives:
In some patients, obstructive sleep apnea (OSA) can be resolved with improvement in pharyngeal patency by sleeping lateral rather than supine, possibly as gravitational effects on the tongue are relieved. Here we tested the hypothesis that the improvement in pharyngeal patency depends on the anatomical structure causing collapse, with patients with tongue-related obstruction and epiglottic collapse exhibiting preferential improvements.
Methods:
Twenty-four OSA patients underwent upper airway endoscopy during natural sleep to determine the pharyngeal structure associated with obstruction, with simultaneous recordings of airflow and pharyngeal pressure. Patients were grouped into three categories based on supine endoscopy: Tongue-related obstruction (posteriorly located tongue, N = 10), non-tongue related obstruction (collapse due to the palate or lateral walls, N = 8), and epiglottic collapse (N = 6). Improvement in pharyngeal obstruction was quantified using the change in peak inspiratory airflow and minute ventilation lateral versus supine.
Results:
Contrary to our hypothesis, patients with tongue-related obstruction showed no improvement in airflow, and the tongue remained posteriorly located while lateral. Patients without tongue involvement showed modest improvement in airflow (peak flow increased 0.07 L/s and ventilation increased 1.5 L/min). Epiglottic collapse was virtually abolished with lateral positioning and ventilation increased by 45% compared to supine position.
Conclusions:
Improvement in pharyngeal patency with sleeping position is structure specific, with profound improvements seen in patients with epiglottic collapse, modest effects in those without tongue involvement and—unexpectedly—no effect in those with tongue-related obstruction. Our data refute the notion that the tongue falls back into the airway during sleep via gravitational influences.
Keywords: Supine position, airway obstruction, epiglottis, sleep apnea.
Statement of Significance
Sleeping lateral rather than supine can have a profound impact on obstructive sleep apnea (OSA) but the improvement in upper airway patency varies from patient to patient for reasons that remain unclear. We show that the improvement in airway patency with lateral positioning depends on the pharyngeal structure impeding airflow: Patients with epiglottic collapse improved markedly and those without tongue-related obstruction (soft-palate/lateral walls) showed modest improvements. Most surprisingly, relief from gravitational effects on the tongue with lateral positioning had no effect on airway patency in patients with tongue-related obstruction.
INTRODUCTION
Obstructive Sleep Apnea (OSA) is characterized by recurrent upper airway collapse1 and is often aggravated by sleeping in the supine position.2 However, there is wide variability between patients in the response to positional changes. The reason for this variability is poorly understood. Some studies suggest that sleeping supine could affect the collapse of particular structures within the airway, such as the tongue base or epiglottis.3–5 However, these studies lacked quantitative measurements of airway size and flow in the different sleeping positions, making it difficult to determine the effect size of positional changes.
In recent years, the large number of drug-induced sleep endoscopy (DISE) studies performed in OSA patients has shown that upper airway obstruction results from the collapse of one or more pharyngeal structures: the soft palate, the lateral pharyngeal walls, the tongue base, and the epiglottis.6,7 Furthermore, imaging studies have shown that gravity can affect upper airway soft tissue structures and their relationship with bony enclosures.8,9 However, the positional effect on pharyngeal structures such as the tongue base and epiglottis was not reported in these studies. It is unknown how lateral sleep affects the tongue position and airway patency when compared to the supine position. Likewise, changes in epiglottic closure due to sleep position have also not been well characterized.
We hypothesized that the improvement in upper airway patency from supine to lateral sleep would be dependent on the structure causing pharyngeal collapse. Specifically, we hypothesized that patients with tongue-related obstruction or epiglottic collapse might be the most amenable to positional changes. This hypothesis was based on the theoretical notion that gravity may have a greater effect on these structures. To test this hypothesis, we evaluated OSA patients in lateral and supine sleep with upper airway endoscopy and concurrent measurements of airflow and epiglottic pressure.
METHODS
Subjects
Patients with OSA as defined by an apnea-hypopnea index (AHI) > 10 events/h were invited to participate in the study. The age range was 21–70 years. Subjects were excluded if they had heart failure, diabetes, or renal insufficiency, or if they were taking medications that could affect upper airway muscle function. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital. Written informed consent was obtained before participation in the study.
Instrumentation
Subjects were instrumented with electrodes for electroencephalography (C4-A1, O2-A1), left and right electrooculography, and submental electromyography for sleep staging. After topical application of a decongestant (oxymetazoline 0.05%) and anesthetic (lidocaine 4%), a 5-French pressure catheter (Millar Instruments, Houston, TX) and a 2.8 mm-diameter pediatric bronchoscope (model BFXP-160F, Olympus, Tokyo, Japan) were inserted through the left and right nostrils, respectively. The pressure catheter was placed at the level of the epiglottis. Subjects breathed via a nasal mask that was connected to a pneumotachometer (Hans-Rudolph, Kansas City, MO) and a differential pressure transducer (Validyne, Northridge, CA) to measure airflow. A modified continuous positive airway pressure (CPAP) device (Pcrit 3000, Philips Respironics, Monroeville, PA) was attached to the mask to hold the airway open when needed to enable visualization of all airway structures. To standardize observations, the position of the airway structures and airflow rates were quantified at the same CPAP levels during supine and lateral sleep. Spike 2 software (Cambridge Electronic Design, Cambridge, England) was used to acquire the physiologic signals and endoscopic images. All signals were captured at a sampling frequency of 500 Hz, and the images were sampled at 30 frames/s.
Protocol
The subjects were asked to sleep in the supine position on a thin pillow with the chin forward. For the lateral position, they were asked to lie in the right lateral decubitus position with the head in a lateral neutral position. The body position was monitored directly by the investigator who stayed inside the room during the study and assured that both postures were constant during data collection. In order to evaluate the different pharyngeal structures causing collapse, the bronchoscope’s tip was initially placed in the nasopharynx above the palate. Several sequences of flow-limited breaths (lack of increase in flow despite decreasing epiglottic pressure) were recorded with the scope in the nasopharynx. The tip was then advanced to the oropharynx to observe the oropharyngeal and hypopharyngeal structures. Once the bronchoscope was placed at each one of the pharyngeal levels, minimal movement was then performed to prevent the subjects from awakening. This process was then repeated in the alternate sleeping position. As many breaths were observed in both sleeping positions and at both pharyngeal levels as possible during the night of study.
Data Analysis
Patients were categorized into two groups according to the position of the tongue base: tongue-related obstruction and non-tongue related obstruction. Tongue-related obstruction was defined when the tongue base was touching or covering the anterior aspect of the epiglottis and thus obliterating the vallecula. The cardinal feature of this group was a posteriorly located tongue during expiration and inspiration. Non-tongue related obstruction was defined when collapse occurred due to the palate or lateral walls without tongue base involvement (the vallecula was clearly visible and the tongue base was not touching the epiglottis). The occurrence of epiglottic collapse was associated with severe airflow obstruction regardless of the location of the tongue base (see description of epiglottic collapse below), and for this reason the presence of epiglottic collapse received its own category. The epiglottis could obstruct the pharynx by either anteroposterior or lateral (folding) movement. This classification was based on the video images of the velopharynx and oropharynx recorded during the natural sleep in both supine and lateral positions. Each patient was classified independently by two investigators blinded to the sleeping position after the recording of the full study. Any discrepancies were resolved by a third investigator.
In order to assess changes in the functional size of the airway due to positional changes, inspiratory peak airflow and minute ventilation from all eligible flow-limited breaths during non-rapid eyes movement (NREM) sleep were analyzed. Pharyngeal pressure was monitored to determine flow-limitation defined by the lack of increase in flow despite decreasing epiglottic pressure. Eligible breaths included those that were free of artifacts and arousals and where the endoscope was in the nasopharynx (above the palate and not touching the airway structures of interest). Particular attention was paid to peak flow during flow limitation because previous studies have suggested that it is a good surrogate measurement of upper airway size and collapsibility.10,11 This concept is derived from the observation that, during flow limitation, peak inspiratory flow is correlated with pharyngeal cross-sectional area at end-expiration of the previous breath.
Statistical Analysis
Descriptive values are presented as the mean ± standard deviation or the median (1st, 3rd quartile) for continuous variables. Demographic variables were compared with a one-way analysis of variance and Chi-square test. A McNemar Chi Square statistic was used to test for changes in the collapse category with changes in position. Because collapse of the epiglottis is intermittent, as described below, the proportion of breaths with epiglottic collapse between the supine and lateral positions were compared using a Wilcoxon signed-rank test. A Wilcoxon signed-rank test was used for comparisons between the inspiratory peak flow and ventilation in different positions. Data analysis was performed using SPSS statistical software (version 17, SPSS Inc., Chicago, IL), and statistical significance was considered when the p value was < .05.
RESULTS
Twenty-four OSA patients (age 53 ± 6 years; 17 men and 7 women) were studied. The subjects’ characteristics are presented in Table 1. We analyzed a total of 886 flow-limited breaths during supine NREM sleep (37 ± 45 breaths per subject) and 1026 flow- limited breaths (43 ± 43 breaths per subject) during lateral NREM sleep. The pharyngeal pressure swings for the breaths in the supine position were −10.7 ± 7.1 cmH2O, and the pharyngeal pressure swings for the breaths in the lateral position were −8.5 ± 4.7 cmH2O. Four subjects required CPAP to hold the airway open enough to visualize the structures involved in collapse. The CPAP used in these patients were 2, 5, 6, and 8 cmH2O. At atmospheric pressure, these four patients exhibited continuously cycling apneas with frequent arousals, which made observation of the collapsing structures difficult. In order to make equal comparisons between supine and lateral sleep, care was taken to study these patients at the same level of CPAP in both positions. Inspiratory peak flow across all patients was 0.22 (0.14–0.35) L/s in supine position, and 0.24 (0.16–0.33) L/s in lateral sleeping position (p < .001). Minute ventilation across all patients was 4.75 (3.19–6.58) L/min in the supine position, and 5.85 (3.95–7.42) L/min in the lateral position (p < .001).
Table 1.
Subjects Characteristics.
| All subjects (n = 24) | Tongue-related obstruction (n = 10) | Non-tongue related obstruction(n = 8) | Epiglottic collapse (n = 6) | p | |
|---|---|---|---|---|---|
| Age (years) | 53.3 ± 6.6 | 54.8 ± 5.9 | 49.9 ± 6.2 | 56.3 ± 7.2 | .14 |
| Sex (M/F) | 17/7 | 5/5 | 6/2 | 6/0 | .09 |
| Neck circumference (cm) | 41.1 ± 3.2a | 40.5 ± 3.2a | 41.2 ± 3.6a | 40.7 ± 3.0 | .61 |
| BMI (kg/m2) | 32.0 ± 5.9 | 33.3 ± 7.3 | 31.3 ± 2.8 | 31.0 ± 6.9 | .69 |
| AHI (events/hour) | 48 ± 28 | 41.5 ± 27.0 | 58.0 ± 33.1 | 45.3 ± 21.7 | .46 |
AHI = apnea-hypopnea index; BMI = body mass index. Data are presented as mean ± standard deviation.
adata not reported for one subject in tongue-related obstruction group and one subject in the non-tongue related obstruction group.
Tongue-Related Obstruction (n = 10)
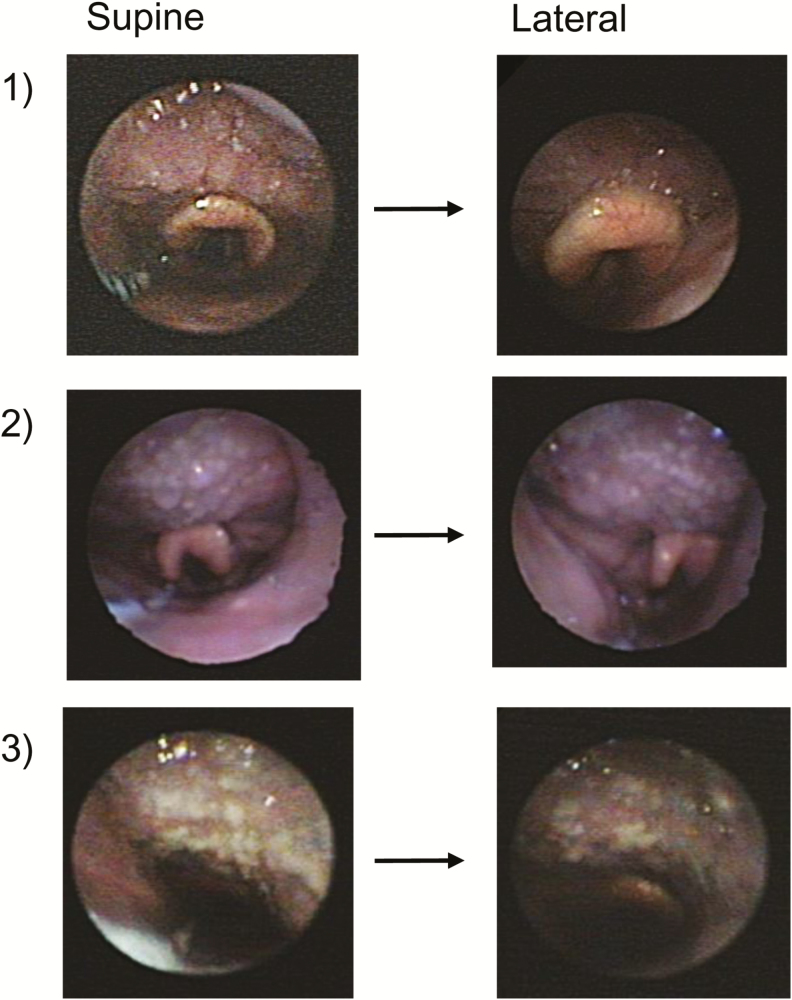
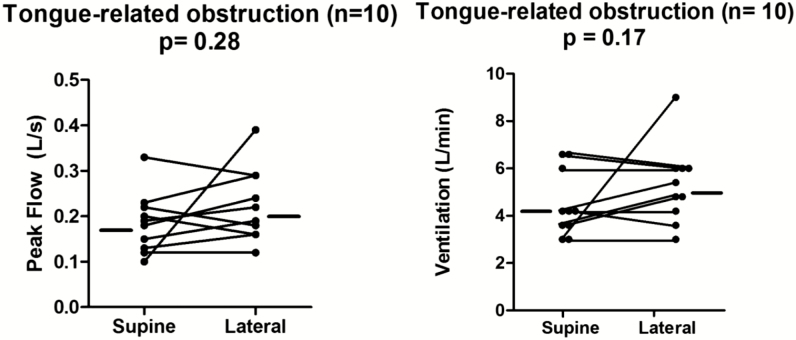
We hypothesized that the tongue base would be affected by gravity and, if posteriorly located in the supine position, would move anteriorly during lateral sleep and be associated with improved airflow. To test this hypothesis, we assessed the position of the tongue endoscopically and airflow parameters in supine and lateral sleep. Contrary to our original hypothesis, we found that in the ten patients with a posteriorly located tongue during supine sleep, the tongue remained posteriorly located in seven patients in lateral sleep (p = 1.0). Note the lack of tongue movement in three representative patients in Figure 1. In two patients in this group, the endoscopic oropharyngeal views during lateral sleep were not adequate for evaluation of the tongue position. Therefore, we did not have adequate information in these two individuals to draw conclusions about the location of the tongue base during lateral sleep. In another patient, we observed an anterior movement of the tongue in the lateral sleeping position compared to supine, allowing clear visualization of the vallecular in the lateral position. None exhibited isolated palatal or lateral walls collapse upon moving to the lateral position (while some patients appeared to narrow in the lateral dimension, the lateral walls never actually touched). In addition to no change in tongue position, there was no improvement in inspiratory peak flow or ventilation. As shown in Figure 2, the inspiratory peak flow increased negligibly (lateral minus supine: 0.03 L/s), and the ventilation did not change (lateral minus supine: 0 L/min). These data demonstrate that neither the tongue position nor the functional size of the airway changes with a change in body position in patients with predominantly tongue related obstruction.
Figure 1.
Endoscopic oropharyngeal views of tongue-related obstruction in three representative subjects. Supine sleep is shown in the left panel and lateral sleep is shown in the right panel. Note that the tongue is touching the epiglottis and obliterating the vallecular in both supine and lateral sleep.
Figure 2.
Peak flow and ventilation did not increase from supine to lateral sleep in patients with tongue-related obstruction. Data presented as individual median values for each patient in both sleeping positions.
Non-Tongue Related Obstruction (n = 8)
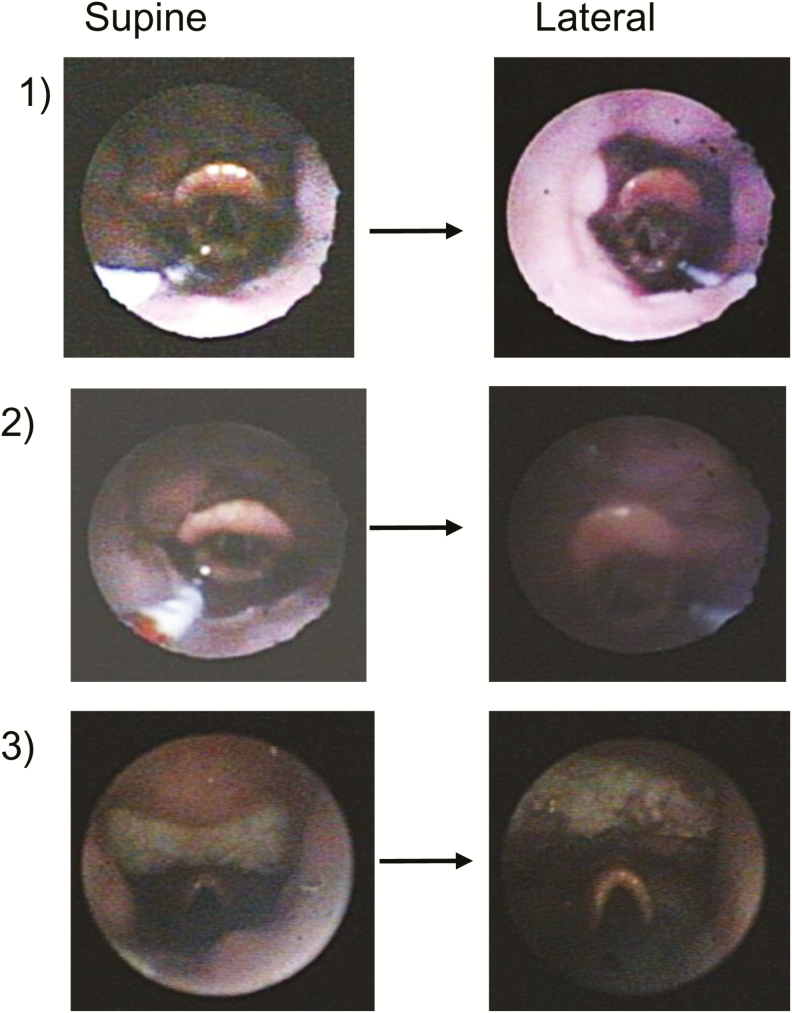
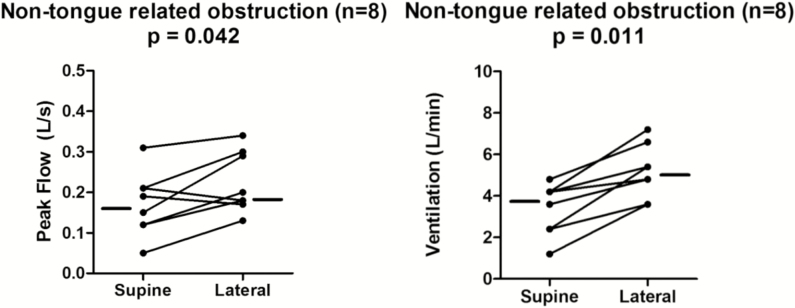
We originally hypothesized that patients without tongue base obstruction, that is, collapse due to the palate and/or lateral walls, would exhibit no improvement (and possibly worsening in the case of lateral walls collapse) in the lateral position. We found that in these patients, the tongue base remained in an “anterior” location during lateral sleep (Figure 3), and the structure causing collapse did not change. All eight patients in this group had non-tongue obstruction during both supine and lateral sleep (p = 1.0). Contrary to our original hypothesis, we found a slight increase in inspiratory peak flow and an even larger increase in ventilation (Figure 4). The inspiratory peak flow increased 0.07 L/s (p = .04), and the ventilation increased 1.5 L/min (p = .01). Hence, in these patients without tongue related obstruction, airway patency tended to increase.
Figure 3.
Endoscopic oropharyngeal views of non-tongue related obstruction in three patients. The tongue is not touching the epiglottis and the vallecular is visible in both positions.
Figure 4.
Peak flow and ventilation increased in lateral sleep among patients without tongue-related obstruction. Data presented as individual median values for each patient in both sleeping positions.
Epiglottic Collapse (n = 6)
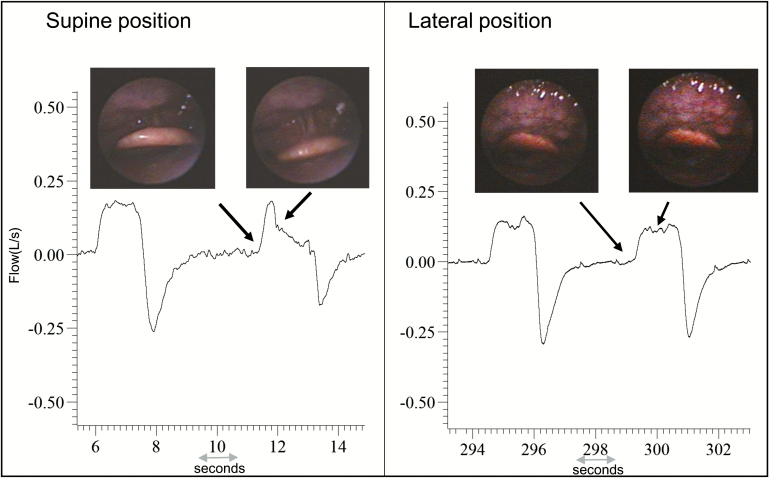
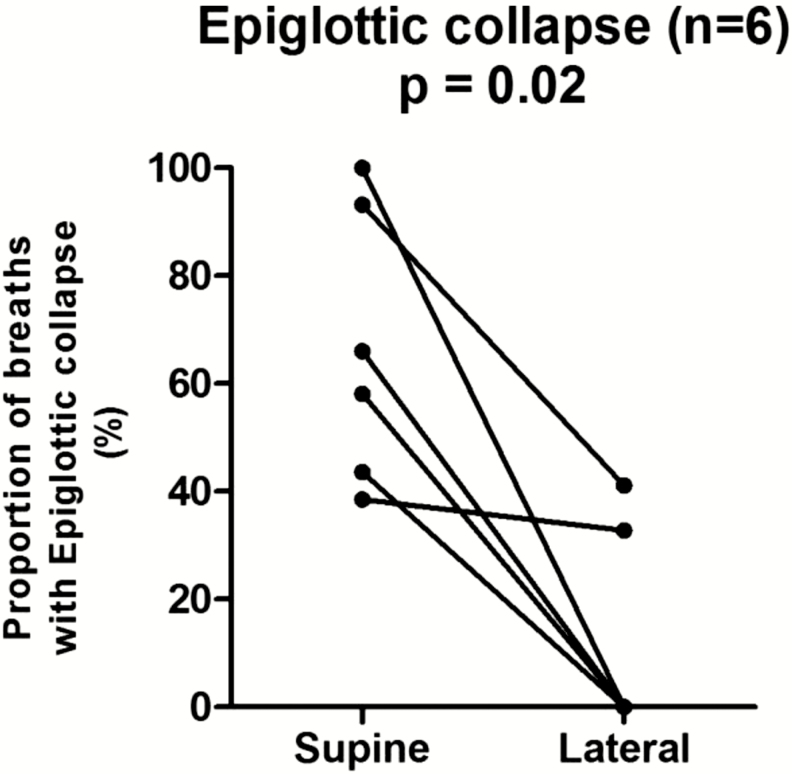
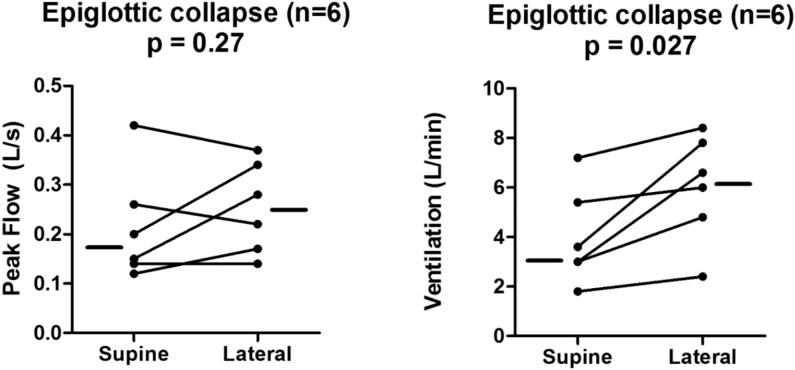
Epiglottic collapse (Figure 5) was placed in a separate category because it is often associated with severe and abrupt changes in flow that, when present, predominate as the mechanism of collapse. This behavior is illustrated in Figure 6, which shows a raw tracing of epiglottic collapse. In the supine position at end-expiration in this patient, the epiglottis is low-hanging and partially covers the laryngeal inlet. During inspiration, the epiglottis closes rapidly and completely. Note also how the epiglottic collapse occurs immediately after a non-flow limited breath; this is a cardinal feature of epiglottic collapse, that is, it is intermittent. To deal with this intermittency, we counted the percentage of breaths exhibiting epiglottic collapse in the supine position and compared it to the percentage of breaths with epiglottic collapse in the lateral position. We found that epiglottic collapse decreased markedly in most patients (from 66.5% ± 25.3% to 12.3% ± 19.2%, see Figure 7). This was supported by objective measurements of ventilation, which increased from 3.3 (2.7–5.8) L/min while supine to 6.3 (4.2–7.9) L/min while lateral (Figure 8). The inspiratory peak flow, on the other hand, did not increase, possibly because epiglottic collapse often occurs abruptly during mid-inspiration and thus does not alter inspiratory peak flow (note in Figure 6 that peak flow is “normal”). Also because of the intermittent nature of the epiglottic collapse, other potential sites of collapse (ie, soft palate, lateral walls) were observed on the breaths when epiglottic collapse did not occur. Two patients with epiglottic collapse also exhibited collapse of the lateral walls without tongue base involvement.
Figure 5.
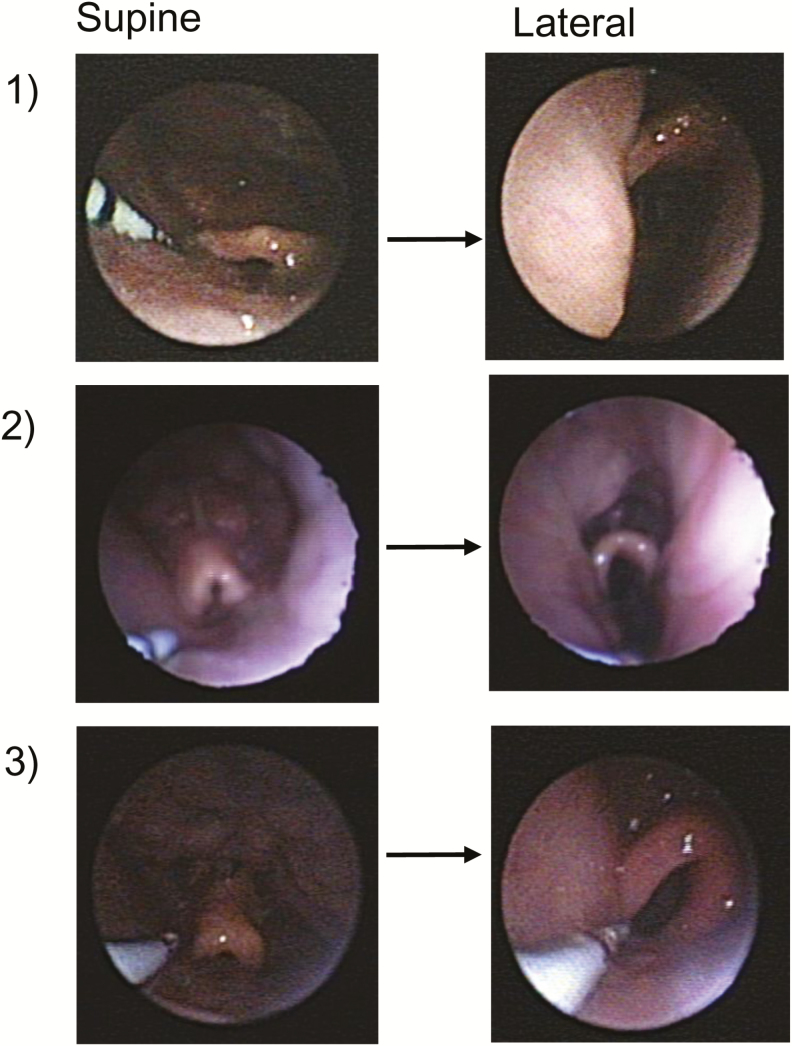
Endoscopic views of epiglottic collapse in three subjects. The epiglottis appeared more posterior and closer to laryngeal inlet during supine sleep than in the lateral position.
Figure 6.
Raw data of a representative subject showing the retrodisplacement of the epiglottis in supine sleep, and inspiratory airflow reduction concomitant to the epiglottic collapse. The epiglottis is less close to the laryngeal inlet, and it is not collapsing in the lateral position. The arrows indicate the epiglottis position at the end-expiration and initial inspiration.
Figure 7.
Reduction in the proportion of breaths exhibiting epiglottic collapse from supine to lateral sleep.
Figure 8.
Ventilation improved from supine to lateral sleep in patients with epiglottic collapse. Data presented as individual median values for each patient in both sleeping positions. .
DISCUSSION
The major findings of the current study were:
Patients with tongue related obstruction did not exhibit a change in tongue position or airflow upon moving onto their side.
Airway patency improves slightly with lateral positioning in patients “without” tongue related obstruction.
Epiglottic collapse improves substantially with a change to the lateral body position during sleep.
The postural differences observed in OSA patients are likely due to some combination of unfavorable upper airway geometry, decreased lung volume, and failure of the dilator muscles to compensate.12 The effect of sleeping position on upper airway soft tissue structures and their relation to bony enclosures has also been investigated with different imaging methods. Walsh et al. performed optical coherence tomography in 11 OSA patients and 11 control subjects during wakefulness.13 The pharyngeal cross sectional area did not change when moving from the supine to the lateral position in both groups. On the other hand, Isono et al. used endoscopy during general anesthesia and reported a larger cross sectional area in the lateral posture in eight OSA patients.14 However, neither of these studies attempted to identify reasons for inter individual differences in response to positional changes. Differences in upper airway shape have also been shown to participate in the genesis of positional airway obstruction.15 For instance, a more elliptical airway shape with reduced anteroposterior diameter in supine posture increases the susceptibility for pharyngeal collapse.13,16 More recently, DISE has been used to evaluate the influence of head position on upper airway obstruction and has shown a decreased frequency of complete anteroposterior collapse at the velum, tongue base, and epiglottis level with lateral rotation of the head.5 Lee et al. performed DISE in 85 patients and reported a decrease in the prevalence of tongue base obstruction when moving from supine (71.1%) to lateral sleep (7.1%).3 However, there are some methodological differences between this paper and our study. First, as a condition for tongue base obstruction, Lee and colleagues used the criteria of a 50% displacement compared to wakefulness. This is problematic because visual estimates of percent collapse are highly subjective. To reduce the subjectivity, we adopted a categorization for airway collapse that considered the position of the tongue base relative to the vallecula and epiglottis during end-expiration. The use of such anatomical landmarks, we believe, makes the categorization criteria more objective. Additionally, we observed the relation between the structures during end-expiration, which could minimize the fact that the airway structures are heavily influenced by the pressure in the pharynx during inspiration. For instance, if the palate collapses in the supine position then this would make the downstream oropharyngeal pressure much more negative during inspiration, which could then pull the tongue into the airway. Had the palate not collapsed (and the negative oropharyngeal pressure not been created), then the tongue may not have collapsed. Thus, one future research direction is to evaluate the relationship between the tongue position and epiglottic pressure.
Our finding that patients who exhibit tongue related obstruction do not show improved airway patency when moving from supine to lateral has several pathophysiological implications. First, it is now unlikely—in patients with oropharyngeal narrowing—that the reason for upper airway collapse while supine is that the tongue simply falls back into the airway during sleep, since the tongue “falls” into the airway during supine sleep to the same exact extent in the lateral position, which is not attributable to gravitational influences. Thus an alternative mechanism is needed for these patients. The muscular hydrostat argument17,18 may provide insight into this phenomenon, whereby the tongue maintains a constant volume (regardless of the forces acting on it ie, gravity, muscle activation). In this case, our data lead us to speculate that an enlarged tongue (thereby posteriorly located) has little space to move into (with position changes/muscle activation) regardless of whether supine or lateral. If the tongue was anteriorly located, it still could be sucked into the airway—but this only happened when the palate collapsed first causing the epiglottic pressure to become very negative (−10 to −15 cmH2O). In one patient, the tongue did seem to “creep” backwards over time (several breaths), but this was difficult to quantify and in the overwhelming majority of the breaths the tongue appeared “anteriorly located.” Additionally, the tongue does not fall back when these patients with non-tongue obstruction move from the lateral to the supine position possibly because the muscles activate to move the tongue forward resisting the collapsing force (as opposed to a large tongue which has little space to move when activated).
Regarding epiglottic collapse, a recent systematic review in adult OSA patients indicated that this occurs more often than previously described from studies performed during wakefulness or with CT imaging, which provides poor visualization of the epiglottis.19 Before the use of DISE to evaluate upper airway obstruction, the prevalence of epiglottic collapse was estimated to be 12% of OSA patients. However, studies performed during (drug-induced) sleep have demonstrated a much higher prevalence. Lan and colleagues studied 64 OSA patients with DISE and found that 30% exhibited complete collapse of the epiglottis.20 Cavaliere et al found that 23% of the 66 patients studied had epiglottis involvement.21 A similar prevalence of 24% was found in Ravesloot’s study of 100 consecutive OSA patients.22 Finally, Golz and colleagues noted that 26% of the 187 patients they studied had epiglottic collapse.23 Similarly, we found epiglottic collapse in 25% of the 24 patients included in our study. Therefore, epiglottic collapse is more common than previously thought.
Research also indicates that epiglottic collapse is difficult to treat. Some studies demonstrate that CPAP may be ineffective in patients who have a severely floppy epiglottis because the pressure may further push the epiglottis into the laryngeal inlet.24–26 The extent to which epiglottic collapse contributes to CPAP failure or non-adherence is not known and is an area of interest for future study. Although surgical resection or trimming of the epiglottis may be an option, no controlled studies have reported the efficacy of this approach, and the potential for side effects such as aspiration are not trivial.27,28 Epiglottic collapse may also be difficult to treat with oral appliances. In the presence of hypopharyngeal obstruction (mainly epiglottic collapse) during DISE, simulation of jaw advancement for prediction of oral appliance therapy outcome showed a tendency towards less successful treatment.29 Furthermore, Kent et al performed DISE in thirty-five consecutive patients with incomplete resolution of OSA following oral appliance therapy. Eleven patients (31.4%) had complete epiglottic obstruction at baseline, and with the oral appliance seven of them (64%) had persistent collapse of the epiglottis.30 Therefore, conventional therapies may not be effective to treat OSA associated with epiglottic collapse.
The results of the current study suggest that epiglottic collapse responds well to positional therapy. Consistent with this finding, two previous studies have suggested that positional therapy is an effective treatment for patients with epiglottic collapse. In a study that compared patients with positional and non-positional OSA, Victores et al. demonstrated that epiglottic collapse in the supine position was twice as frequent among the positional (64%) as compared with the non-positional patients (36%).4 In that study, epiglottic collapse in positional OSA patients improved with lateral positioning. Safiruddin et al. also showed that lateral head rotation was associated with improvement in epiglottic collapse as compared to supine sleep.5 However, both studies were based on qualitative DISE findings. Our study measured airflow and thus adds objective evidence of airway anatomical changes. From these data, we speculate that the epiglottis is affected by gravity and is therefore influenced by position changes.
Although positional therapy is an inexpensive therapeutic choice, it is rarely prescribed for OSA.31 There may be several reasons for this. First, for a long time, techniques such as the “tennis-ball method” were the only available procedures for avoiding supine sleep. Oksenberg et al. tested the tennis ball technique in 12 positional OSA patients and showed a reduction of supine sleep duration from 79% to 12% with a concomitant reduction of the AHI from 47 to 18 events/h.32 However, a subsequent study compared a modified tennis ball technique to CPAP in 20 positional OSA patients and found that CPAP was more effective at reducing the AHI.33 The discomfort related to this method was a barrier for its use and resulted in poor long-term compliance, with less than 10% of patients reporting continued use 2.5 years after prescription.34 However, new devices specifically designed to prevent supine sleep have been developed. Bignold et al. tested a position monitoring and supine alarm device. It recorded sleep position accurately and reduced both the supine time (from 19% to 0.4%) and AHI (25 events/h to 13 events/h).35 Another device which is worn around the chest and vibrates when the patient lies supine was associated with good compliance (64% using more than 4 h/night) over 6 months.36,37 Finally, a neck-worn device designed to restrict supine sleep showed benefits by improving the AHI (average reduction of 69%) and sleep architecture (decreased number of arousals and increased N2).38 Therefore, more comfortable and effective devices may be used for positional therapy.
This study has several limitations. First, due to the invasiveness of the study, the sample size was fairly small. Nevertheless, the different collapsing groups were equally well represented and clear changes in the outcome variables were observed during natural sleep endoscopies. Second, a complete set of polysomnographic parameters, including the AHI could not be obtained for all patients due to the complexity of the protocol instrumentation. However, airflow and ventilation measurements were obtained under both study conditions, allowing comparison of changes in the functional size of the airway. Third, the instruments themselves could have affected the site of collapse and the flow rates. However, we believe this is unlikely because when the scope is in the velopharynx, it is not touching any of the airway structures. In addition, while the scope was touching the palate when it was placed in the oropharynx, the flow rates from these breaths were excluded from analysis. Finally, we note that a previous study has found that the upper airway is not altered by the presence of a catheter in the airway.39
In conclusion, improvement in pharyngeal patency with sleeping position is structure specific. Our findings suggest that the tongue may not play as important a role as previously thought. In particular, this study shows that lateral positioning for epiglottic collapse is quite effective. Therefore, if CPAP alternatives are being considered for these patients, then positional therapy is a potential option.
FUNDING
This work was performed at Brigham and Women’s Hospital and was supported by the National Institutes of Health grants R01 HL102321 and P01 NIH HL095491, and also by Harvard Catalyst (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1TR001102). MM and PRG were supported by Capes Foundation, Ministry of Education of Brazil. SAS was supported by the American Heart Association (11POST7360012, 15SDG25890059), National Health and Medical Research Council of Australia (1053201, 1035115) and RG Menzies Fellowship, and an American Thoracic Society Foundation Unrestricted Grant. LTM is supported by the American Heart Association (15POST25480003).
DISCLOSURE STATEMENT
MM, PRG, SAS, AA, and CDM declare no conflicts of interest. LTM serves as consultant for Novion pharmaceuticals Inc. DPW receives salary from Apnicure Inc. and serves as a consultant for Philips Respironics and Night Balance. AW receives research support from Philips Respironics.
ACKNOWLEDGMENTS
The authors would like to acknowledge Lauren Hess for her technical support.
REFERENCES
- 1. Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010; 90(1): 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McEvoy RD, Sharp DJ, Thornton AT. The effects of posture on obstructive sleep apnea. Am Rev Respir Dis. 1986; 133(4): 662–666. [DOI] [PubMed] [Google Scholar]
- 3. Lee CH, Kim DK, Kim SY, Rhee CS, Won TB. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position: a DISE study. Laryngoscope. 2015; 125(1): 248–254. [DOI] [PubMed] [Google Scholar]
- 4. Victores AJ, Hamblin J, Gilbert J, Switzer C, Takashima M. Usefulness of sleep endoscopy in predicting positional obstructive sleep apnea. Otolaryngol Head Neck Surg. 2014; 150(3): 487–493. [DOI] [PubMed] [Google Scholar]
- 5. Safiruddin F, Koutsourelakis I, de Vries N. Analysis of the influence of head rotation during drug-induced sleep endoscopy in obstructive sleep apnea. Laryngoscope. 2014; 124(9): 2195–2199. [DOI] [PubMed] [Google Scholar]
- 6. Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011; 268(8): 1233–1236. [DOI] [PubMed] [Google Scholar]
- 7. Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope. 2014; 124(3): 797–802. [DOI] [PubMed] [Google Scholar]
- 8. Ono T, Otsuka R, Kuroda T, Honda E, Sasaki T. Effects of head and body position on two- and three-dimensional configurations of the upper airway. J Dent Res. 2000; 79(11): 1879–1884. [DOI] [PubMed] [Google Scholar]
- 9. Saigusa H, Suzuki M, Higurashi N, Kodera K. Three-dimensional morphological analyses of positional dependence in patients with obstructive sleep apnea syndrome. Anesthesiology. 2009; 110(4): 885–890. [DOI] [PubMed] [Google Scholar]
- 10. Isono S, Feroah TR, Hajduk EA, Brant R, Whitelaw WA, Remmers JE. Interaction of cross-sectional area, driving pressure, and airflow of passive velopharynx. J Appl Physiol. 2014:851–859. [DOI] [PubMed] [Google Scholar]
- 11. Azarbarzin A, Sands SA, Montemurro LT, et al. Estimation of pharyngeal collapsibility during sleep by peak inspiratory aiflow. Sleep. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joosten SA, Edwards BA, Wellman A, et al. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep. 2015; 38(9): 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh JH, Leigh MS, Paduch A, et al. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. Sleep. 2008; 31(11): 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology. 2002; 97(4): 780–785. [DOI] [PubMed] [Google Scholar]
- 15. Joosten SA, O’Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014; 18(1): 7–17. [DOI] [PubMed] [Google Scholar]
- 16. Joosten SA, Sands SA, Edwards BA, et al. Evaluation of the role of lung volume and airway size and shape in supine-predominant obstructive sleep apnoea patients. Respirology. 2015; 20(5): 819–827. [DOI] [PubMed] [Google Scholar]
- 17. Gilbert RJ, Napadow VJ, Gaige TA, Wedeen VJ. Anatomical basis of lingual hydrostatic deformation. J Exp Biol. 2007; 210(pt 23): 4069–4082. [DOI] [PubMed] [Google Scholar]
- 18. Kairaitis K. Is the pharynx a muscular hydrostat? Med Hypotheses. 2010; 74(3): 590–595. [DOI] [PubMed] [Google Scholar]
- 19. Torre C, Camacho M, Liu SY, Huon LK, Capasso R. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope. 2016; 126(2): 515–523. [DOI] [PubMed] [Google Scholar]
- 20. Lan MC, Liu SY, Lan MY, Modi R, Capasso R. Lateral pharyngeal wall collapse associated with hypoxemia in obstructive sleep apnea. Laryngoscope. 2015; 125(10): 2408–2412. [DOI] [PubMed] [Google Scholar]
- 21. Cavaliere M, Russo F, Iemma M. Awake versus drug-induced sleep endoscopy: evaluation of airway obstruction in obstructive sleep apnea/hypopnoea syndrome. Laryngoscope. 2013; 123(9): 2315–2318. [DOI] [PubMed] [Google Scholar]
- 22. Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope. 2011; 121(12): 2710–2716. [DOI] [PubMed] [Google Scholar]
- 23. Golz A, Goldenberg D, Westerman ST, et al. Laser partial epiglottidectomy as a treatment for obstructive sleep apnea and laryngomalacia. Ann Otol Rhinol Laryngol. 2000; 109(12 pt 1): 1140–1145. [DOI] [PubMed] [Google Scholar]
- 24. Verse T, Pirsig W. Age-related changes in the epiglottis causing failure of nasal continuous positive airway pressure therapy. J Laryngol Otol. 1999; 113(11): 1022–1025. [DOI] [PubMed] [Google Scholar]
- 25. Shimohata T, Shinoda H, Nakayama H, et al. Daytime hypoxemia, sleep-disordered breathing, and laryngopharyngeal findings in multiple system atrophy. Arch Neurol. 2007; 64(6): 856–861. [DOI] [PubMed] [Google Scholar]
- 26. Dedhia RC, Rosen CA, Soose RJ. What is the role of the larynx in adult obstructive sleep apnea? Laryngoscope. 2014; 124(4): 1029–1034. [DOI] [PubMed] [Google Scholar]
- 27. Sorrenti G, Piccin O, Mondini S, Ceroni AR. One-phase management of severe obstructive sleep apnea: tongue base reduction with hyoepiglottoplasty plus uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 2006; 135(6): 906–910. [DOI] [PubMed] [Google Scholar]
- 28. Toh ST, Han HJ, Tay HN, Kiong KL. Transoral robotic surgery for obstructive sleep apnea in Asian patients: a Singapore sleep centre experience. JAMA Otolaryngol Head Neck Surg. 2014; 140(7): 624–629. [DOI] [PubMed] [Google Scholar]
- 29. Vroegop AV, Vanderveken OM, Dieltjens M, et al. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res. 2013; 22(3): 348–355. [DOI] [PubMed] [Google Scholar]
- 30. Kent DT, Rogers R, Soose RJ. Drug-induced sedation endoscopy in the evaluation of osa patients with incomplete oral appliance therapy response. Otolaryngol Head Neck Surg. 2015; 153(2): 302–307. [DOI] [PubMed] [Google Scholar]
- 31. Ravesloot MJ, van Maanen JP, Dun L, de Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath. 2013; 17(1): 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: A 6-month follow-up study. Laryngoscope. 2006; 116(11): 1995–2000. [DOI] [PubMed] [Google Scholar]
- 33. Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the ‘tennis ball technique’ versus nCPAP in the management of position-dependent obstructive sleep apnoea syndrome. Respirology. 2008; 13(5): 708–715. [DOI] [PubMed] [Google Scholar]
- 34. Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009; 5(5): 428–430. [PMC free article] [PubMed] [Google Scholar]
- 35. Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011; 7(4): 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013; 17(2): 771–779. [DOI] [PubMed] [Google Scholar]
- 37. van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014; 37(7): 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014; 10(8): 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maddison KJ, Shepherd KL, Baker VA, et al. Effects on upper airway collapsibility of presence of a pharyngeal catheter. J Sleep Res. 2015; 24(1): 92–99. [DOI] [PubMed] [Google Scholar]