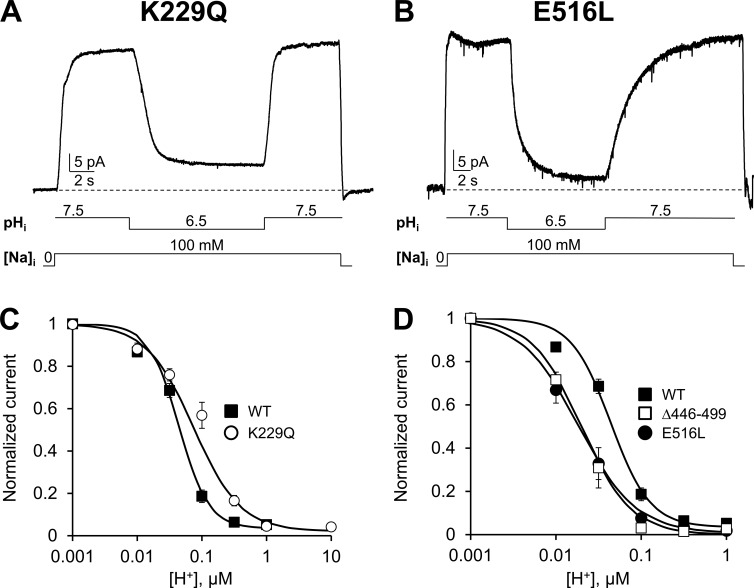
Figure 2.
Effects of Na+ and Ca2+ regulation on NCX pH sensitivity. (A and B) Examples of outward ionic currents recorded from oocytes expressing the indicated mutants. Exchanger K229Q lacks Na+-dependent inactivation (Matsuoka et al., 1997), whereas mutant E516L is not regulated by cytoplasmic Ca2+ (Chaptal et al., 2009). Both mutants retain proton sensitivity indicating that pH regulation can occur in the absence of Na+ or Ca2+ regulation. (C) The dose–response curve for cytoplasmic protons for exchanger K229Q (○) is shown and compared with WT (measured at steady-state, ■). Removal of Na+-dependent inactivation (K229Q) slightly decreases NCX sensitivity to protons and alters the cooperativity of the binding. (D) The pH dependency of steady-state current for mutants E516L (●) and Δ446–499 (□) is shown and compared with WT (■). Disruption of key Ca2+ coordinating sites within CBD1 (Δ446–499) and CBD2 (E516L) does not affect the sensitivity of NCX to cytoplasmic protons. The decrease in the apparent affinity for protons observed is not significant. Error bars represent SEM.