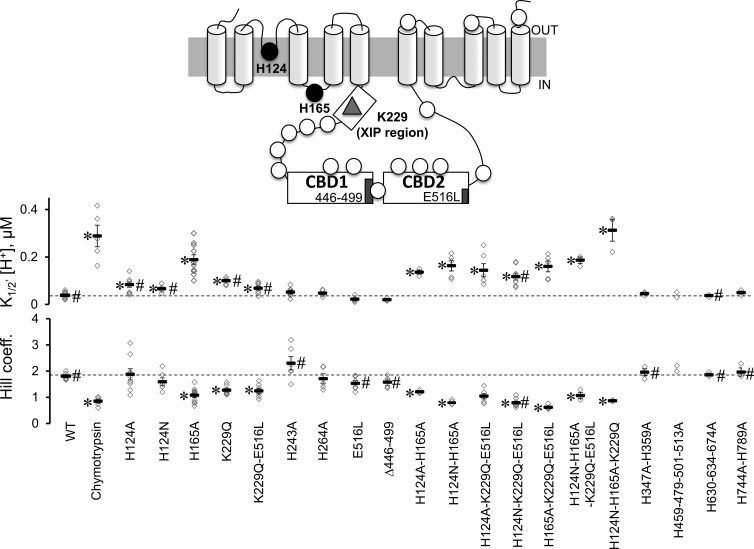
Figure 4.
Proton sensitivity of His mutants. The topology of mammalian NCX is shown as predicted by Ren and Philipson (2013). The approximate position of the 18 histidines (circles), Lys 229 (triangle), the two regulatory Ca2+-binding domains (CBD1 and CBD2) and the XIP region are shown. His residues were mutated to Ala, and the sensitivities of the NCX mutants to cytoplasmic protons were measured at steady state. Normalized currents were plotted as a function of proton concentration to extrapolate the apparent affinity for each single experiment. The number of experiments used for each exchanger mutant is shown with the corresponding mean value ± standard error. Values statistically different after a Bonferroni post hoc test from WT are marked with an asterisk (*, P < 0.0025), whereas values statistically different from WT after chymotrypsin deregulation are marked with # (P < 0.0025). Because of the limited number of experimental observations, we excluded mutant H459-479-501-513A from the multiple comparison procedure and reported the values obtained from the single experiments. Note that all exchangers including mutation of either His 124 or His 165 have decreased sensitivity to protons.