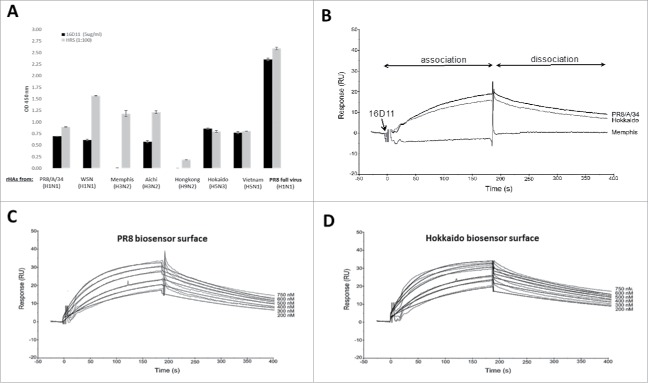
Figure 7.
HA cross-reactivity and binding affinity of 16D11 hu-mAb. (A) Binding of 16D11 hu-mAb to rHA proteins from influenza virus heterotypes in ELISA. Shown are duplicate rHAs-coated wells and+/- SD for 99% confidence. Signal-to-noise background of the anti-human IgM-HRP secondary antibody (0D 450 nm = 0.045 average) has been subtracted from each sample. PR8 virus-coated wells and repository human sera (HRS) were used as controls. (B) SPR comparative binding of 16D11 hu-mAb at 200 nanoMoles to rHA proteins of PR8/a/34 virus (black), Hokkaido virus (grey) and Memphis virus (red). 16D11 injection point and association and dissociation phases are indicated. Representative Sensograms of different concentrations of 16D11 hu-mAbs across biosensor surfaces coupled to rHA protein of PR8 virus (C) and Hokkaido virus (D) at 30 ml/min and 25°C. Sensograms were analyzed using a simultaneous fit algorithm (BIAevaluation 3.1) to calculate the kinetic parameters and binding affinities (as shown in Table 1). SPR sensograms for each response are shown as gray lines whilst fit analyses are shown as black lines.