Figure 5. AFD to AIY connectivity actuates the thermal preference behavior.
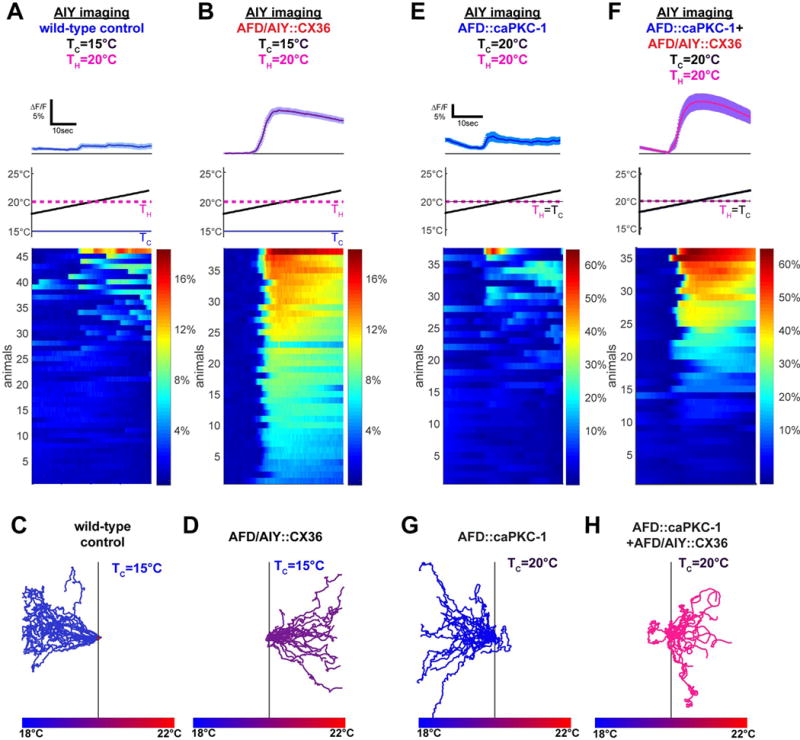
(A) Wild-type animals tested after adaptation above cultivation temperature (TC=15°C<TH=20°C) have weak and infrequent AIY responses near TH (9/46 response penetrance, TS=19.0°C +/−0.7°C). (B) Under the same training conditions, ectopic expression of the gap junction protein CX36 in AFD and AIY (AFD/AIY∷CX36) produces responses in AIY that mirror AFD responses (38/38 response penetrance, TS=19.0°C +/−0.2°C), suggesting this manipulation creates a strong positive coupling between AFD and AIY (see response kinetic analysis and quantification in Figure S5) (C) Worms cultivated at TC=15°C perform negative thermotaxis on a thermal gradient centered at 20°C. (D) Expression of the gap junction protein CX36 in both AFD and AIY produces worms that perform positive thermotaxis under the same conditions. (E) Expression of a constitutively active form of PKC-1 in AFD reduces AIY response frequency when TC=TH=20°C (13/37 response penetrance, T S=19.2°C +/−0.5°C). (F) AFD/AIY∷CX36 increases AIY response frequency even in the presence of the AFD∷caPKC-1 transgene (24/36 response penetrance, TS=19.3°C +/−0.2°C). Because of genetic linkage between the AFD∷caPKC-1 transgene and the AFD∷CX36 transgene, experiments in F (and H) were performed with unstable extrachromosomal lines as described in the Methods (unlike experiments in B and D, which were performed with an integrated CX36 transgenes). (G–H) In contrast to worms expressing constitutively active PKC-1 in AFD, which perform negative thermotaxis when TC=20°C as shown in G, expression of CX36 in AFD and AIY suppresses the phenotype of AFD∷caPKC-1 animals and results in positive thermotaxis as shown in H. For all experiments with CX36, see Figure S5 for corresponding controls of expression of hemichannels and AFD thermosensory responses. Error bars denote SEM.
