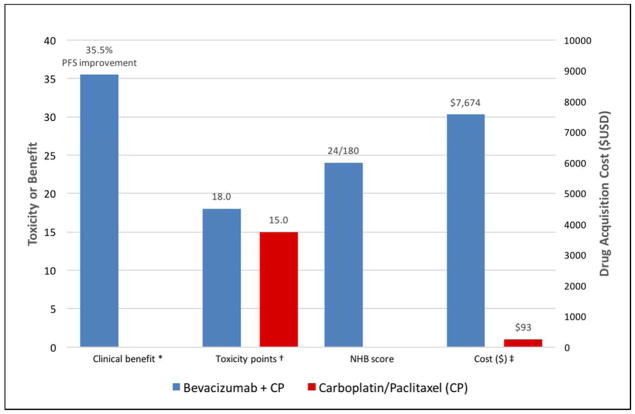
Figure 1.
Value snapshot illustrating ASCO’s comparison of clinical benefit, toxicity, NHB, and cost of novel therapy versus standard therapy. Comparison of bevacizumab + carboplatin/paclitaxel versus carboplatin/paclitaxel alone (GOG 218)
PFS = progression free survival; NHB = Net Health Benefit; CP = Carboplatin/Paclitaxel; USD = 2016 United States dollars
* In accordance with ASCO’s value snapshot, clinical benefit is presented as a percent (%) reduction in risk of progression during the study period based on the hazard ratio (HR) for progression free survival (PFS). Clinical benefit calculations involving a HR rely on the following formula: (1-HR) × 100. For PFS, a multiplier of 0.8 is also applied to this formula.
† The difference in toxicity is graphically shown as the difference in the total point score based on ASCO’s toxicity calculations. ASCO’s revised framework applies different importance weights to all grade 1 or 2 versus grade 3 or 4 adverse events. Toxicity data in GOG 218 reported combined select grade ≥2 adverse events (including grade 2 GI and hypertensive events); therefore, we assigned importance weights based on ASCO’s weighting of grade 3 or 4 adverse events: 1.5 points for each grade 3 or 4 adverse event with <5% frequency; and 2.0 points for each toxicity with a ≥5% frequency. In accordance with ASCO’s framework, ‘laboratory only’ toxicities were excluded. A toxicity score of 18 was calculated for CP + B compared to a score of 15 for CP alone; and a percent difference was calculated and multiplied by 20 in accordance with ASCO’s toxicity calculation. Given CP+B had a higher frequency of toxicities versus CP alone, the overall toxicity score was negative: −4.
‡ The cost estimates are based on Medicare J-code reimbursements; and calculated for a 170cm/70kg female patient with a creatinine of 0.8.