Abstract
Bullous pemphigoid is rare in children and even rarer in infants. By presenting two cases of bullous pemphigoid related by their temporal proximity with a previous episode of vaccination, it will be carefully discussed if a relationship is or is not possible. Our final conclusion is that the association is mostly a myth rather than a reality and bullous pemphigoid is not a contraindication to continue with the normal vaccination schedule of infants. It is important to know about this clinical entity in order to perform adequate treatment that avoids any worsening or future relapse of this disease.
Introduction
Bullous pemphigoid (BP) is an autoimmune bullous condition usually related to the elderly, being rarer in childhood. Some of these cases have been related with a previous episode of vaccination by their temporal proximity. In this article we report two cases of infant BP related in time with a previous episode of vaccination and a review of the literature defining the clinical manifestation of BP and discuss the evidence and the real importance in clinical practice of this relationship “vaccination BP”.
Cases
Case 1
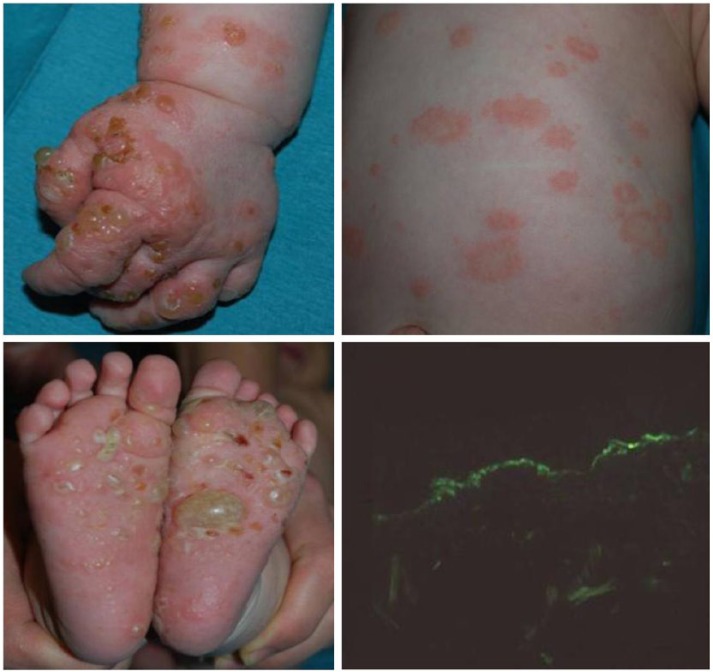
A 3-month-old healthy male infant came to our attention because of a generalized cutaneous rash with blistering lesions on his hands and feet. Annular edematous and erythematous lesions appeared on the trunk and developed 2 days after the first hexavalent vaccination (diphtera anatoxin, polio, Haemophilus influenzae B, Hepatitis B, and pneumococcus). Vesicles and tense bullae on hands and feet were observed 7 days after the first lesions (Figure 1). Mucous membranes were not involved. A skin biopsy of a bulla was performed and treatment with deflazacort 1 mg/kg/day was empirically started, in the suspect of a bullous pemphigoid. Histological examination and direct immunofluorescence study confirmed the initial diagnosis showing a sub-epidermal blister and a linear staining of IgM and C3 along the basement membrane zone, while IgG were not detectable. The immunosorbent assay (commercial ELISA, Biobest Laboratories Ltd.) showed positive values of IgG BP180 (208.33 U/mL) and BP230 1.20 U/mL. Serological testing of the mother’s serum was negative. Therefore systemic steroid treatment was continued for a total of 5 months with a rapid resolution of the lesions. No relapse was observed at successively vaccinations.
Figure 1.
Tensed bullae on the hands and feet and urticarial plaques on the trunk. Direct immunofluorescence with a linear staining of C3 along the basement membrane zone.
Case 2
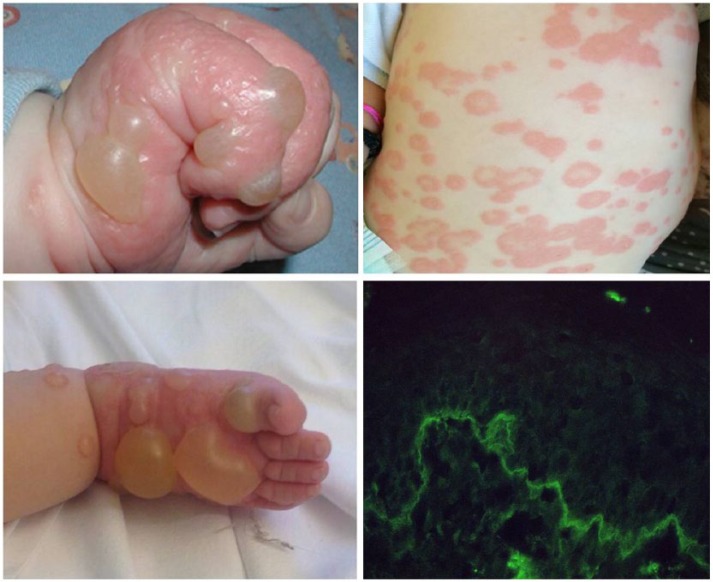
A 6-month-old otherwise healthy boy referred with a 4-day history of bilateral itchy vesiculo-bullous lesions appeared suddenly on the leg, arms neck, feet, and hands. Erythematous and infiltrated plaques developed over the back, chest, and abdomen (Figure 2). No oral mucosal involvement was seen. Blood analysis and infectious serology were normal except for an elevated eosinophilic leukocytosis (15.4%). Clinical history revealed that the first lesions started 3 days after the second administration of the rotavirus vaccine. At the same time no similar skin reactions had appeared after the first and second routine injection of the hexavalent vaccination. A skin biopsy from one of the bullous lesions showed a sub-epidermal blister with a mixed inflammatory cell infiltrate and abundant eosinophils. A direct immunofluorescence showed a linear deposition of IgG and C3 at the dermo-epidermal junction. Also a small linear deposition of IgA was evident. Enzyme-linked immunosorbent assay (commercial ELISA, Biobest Laboratories Ltd.) for BP180 was positive (181 U/mL) while BP230 was negative (5.4 U/mL). A diagnosis of BP was made and the child was started with prednisone at an initial dose of 2 mg/kg/day then reduced to 0.5 mg/kg/day after 2 weeks. Although improvement was immediate with regression of the bullous lesions and the erythematous plaques on the abdomen, a new arising of blisters appeared on the arms and prednisone 1 mg/kg/day was repeated and continued for 1 month, by which time the signs of BP had disappeared. No relapses was observed at successively vaccinations.
Figure 2.
Large blisters on the hands and feet and widespread urticarial plaques on the trunk. Direct immunofluorescence with a linear deposition of IgG at the dermoepidermal junction.
Discussion
BP is rare in children and even rarer in infants. There have been 21 cases reported of BP related to vaccine administration (including our two patients), of which only 12 cases occurred in infants younger than 6 months of age. The sex proportion found was approximately the same (11M:10F) with a mean age at presentation of 3.5 months. The latency or period from vaccination to clinical manifestation was 7.5 days (range, 5 hours–3 weeks).1,2
Remarkably clinical manifestation of infantile BP (< 1 year) differs from childhood BP (>1 year): infant PB usually shows a high frequency of palmo-plantar and facial involvement while genital and mucosal areas are rarely affected. The generalization of the lesions is eventually a subsequent event. Childhood BP is not quite different from adults BP with a possible mucosal involvement and a possible generalization of the lesions, lacking the accentuation of the acral lesions typical of infant PB. In infants, urticarial-like lesions are reported in most cases of PB post vaccination, alone or rarely in conjunction with bullous elements. At the same time Fuertes et al.3 reported a case of PB not following vaccination with urticarial plaques on limbs and trunk. Otherwise Merida et al.4 reported a case of PB post vaccination manifesting only with bullous lesions on palm and soles. Therefore it seems that urticaria-like lesions are not an exclusive feature of PB after vaccine administration, but that they are most frequent in these patients compared to the other PB not following any vaccination. Furthermore these types of lesions are most frequent in infant PB <1 year and they usually appear immediately in association with the bullous lesions on the palm and soles, while in childhood PB and adults these types of lesions, if present, are characteristic of the pre-bullous phase of PB. Infant PB also has an excellent prognosis and resolves rapidly after the initiation of the treatment with adequate response with topical and/or systemic corticoid.5 Some cases of BP have been reported where corticoids were not sufficient and other drugs such as immunoglobulins2 or omalizumab3 have been necessary to control the disease.
The possible relationship between vaccination and BP is still unclear. There are some aspects that may lead to think to a possible connection:
Interval time between vaccination administration and onset of clinical manifestations is narrow;
Cases of recurrence after a new dose of vaccination have been described;6,7
Higher incidence in infants (<1 year), when vaccinations are more frequently performed, and it is probably something going to happen, as the Romans said “post hoc propter hoc”.
On the other hand, in our opinion, the arguments against the existence of a true relationship prevail:
Vaccination in infants is a usual and daily practice in developed countries while cases of BP reported in infants are really limited;
Temporal association has been argued as a fact supporting the relationship between BP and vaccination. Although a relative narrow interval has been reported, vaccination schedule in the first year of life presents a high rate of vaccine administrations, with an average of one vaccination every 1–2 months for the first 6 months. It is therefore not difficult to find a temporal association;
The lack of similarities between the structure of the implicated vaccines and the relevant basement membrane proteins led to believing the unlikely autoimmune hypothesis of infant PB post vaccination, with the mechanism of blister formation induced by autoantibodies against the basement membrane zone;
A vertical transference of antibodies from the mother seems unlikely since in all the cases of PB reported in the literature where the mother’s serum was tested, it did not show circulating antibasement membrane zone antibodies.
It exists a theoretical basis for supporting the vaccination as an immunological stimulus, in fact, vaccination has been related to autoimmune phenomena such as the appearance of DNA antibodies, localized disorders such as reactive arthritis, and systemic diseases such as immune thrombocytopenia purpura or systemic lupus erythematosus.8 According to recent studies by Lo Schiavo et al.,9 it is worth mentioning the possible inflammatory cascade activation mediated by Th17/IL17 pathway. Maybe the trauma caused by the vaccine injection led to Th17 cell activation with increased of IL-17 which is able to release pro-inflammatory cytokines and proteolytic enzymes, and recruit and activate neutrophils, which by itself may result in blister formation. Perhaps some genetically predisposed infants maybe more sensitive to the stimulus.
However, regarding infantile BP, the reality is that there is little evidence in any sense and demonstration of a true relationship is difficult to prove.
Beyond the immunological discussion, pediatricians should be aware that this possible relationship is not significant from a practical point of view. BP in infants has showed a good response to systemic corticoids and generally there are no recurrences. In a few cases where there was a recurrence after a new dose, the manifestation was less intense and with good response to treatment.
Conclusions
Our final conclusion is that the association between BP and vaccination is most likely a myth rather than a reality and BP is not a contraindication to continue with the normal vaccination schedule of infants. Obviously it is important to know this clinical entity in order to perform adequate treatment and avoid any worsening or future relapse of this disease.
Acknowledgments
We thank Dr. Bianchi and Dr. Del Bianco for the support with the laboratory research and immunofluorescence tests.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Waisbourd-Zinman O, Ben-Amitai D, Cohen AD, et al. (2008) Bullous pemphigoid in infancy: Clinical and epidemiologic characteristics. Journal of the American Academy of Dermatology 58: 41–48. [DOI] [PubMed] [Google Scholar]
- 2. Amos B, Deng JS, Flynn K, et al. (1998) Bullous pemphigoid in infancy: Case report and literature review. Pediatric Dermatology 15: 108–111. [DOI] [PubMed] [Google Scholar]
- 3. Fuertes I, Luelmo J, Leat L, et al. (2010) Refractory childhood pemphigoid successfully treated with rituximab. Pediatric Dermatology 30: e96–97. [DOI] [PubMed] [Google Scholar]
- 4. Mérida C, Martínez-Escribano JA, Frías JF, et al. (2005) Bullous pemphigoid in an infant after vaccination. Actas Dermo-Sifiliográficas 96: 255–257. [DOI] [PubMed] [Google Scholar]
- 5. Barreau M, Stefan A, Brouard J, et al. (2012) Infantile bullous pemphigoid. Annales de Dermatologie et Vénéréologie 139: 555–558. [DOI] [PubMed] [Google Scholar]
- 6. Valdivielso-Ramos M, Velázquez D, Tortoledo A, et al. (2011) Infantile bullous pemphigoid developing after hexavalent, meningococcal and pneumococcal vaccinations. Anales de Pediatría 75: 199–202. [DOI] [PubMed] [Google Scholar]
- 7. Fisler RE, Saeb M, Liang MG, et al. (2003) Childhood bullous pemphigoid: A clinicopathologic study and review of the literature. American Journal of Dermatopathology 25: 183–189. [DOI] [PubMed] [Google Scholar]
- 8. Salemi S, D’Amelio R. (2010) Could autoimmunity be induced by vaccination? International Reviews of Immunology 29: 247–269. [DOI] [PubMed] [Google Scholar]
- 9. Lo Schiavo A, Ruocco E, Brancaccio G, et al. (2013) Bullous pemphigoid: Etiology, pathogenesis, and inducing factors: Facts and controversies. Clinics in Dermatology 31: 391–399. [DOI] [PubMed] [Google Scholar]