Abstract
Sirtuins (SIRT) have been regarded as culprits in the pathogenesis of various diseases. Their exact role has not been explained. This study aimed to assess the expression of SIRT1, SIRT6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ in psoriatic patients. Thirty psoriatic patients and 22 controls were enrolled. Clinical examination and Psoriasis Area and Severity Index (PASI) were obtained. Two skin biopsies (lesional, peri-lesional) and one from controls were obtained. Tissue levels of SIRT1, SIRT6, TNF-α, and IFN-γ were measured using ELISA. SIRT1 was significantly lower in lesional skin with gradual increase in perilesional followed by control skin (P <0.001). SIRT6, TNF-α, and IFN-γ were significantly higher in lesional than perilesional and control skin (P <0.001). Significant positive correlations were found between SIRT1 and TNF-α, IFN-γ and between SIRT6 and TNF-α in peri-lesional skin. SIRT1 and SIRT6 are potentially involved in the pathogenesis of psoriasis. Modulating their action could offer a novel therapy for such disease.
Keywords: Psoriasis, Pathogenesis, Sirtuins 1 and 6, TNF-α, IFN-γ
Introduction
In recent years, the Sirtuins family (SIRT) has shown their involvement in multiple cellular pathways related to aging, inflammation, epigenetics, cancer, and a variety of cellular functions including cell cycle, DNA repair, and proliferation, positioning them as postulated perpetrators in different diseases including psoriasis.1 The exact role played by these proteins in such diseases has not been clearly explained.2
It has been proposed that SIRT1 and SIRT6 interact with NF-Кβ via different mechanisms.3 SIRT1-induced deacetylation of NF-Кβ inhibits the latter mediated transcription of tumor necrosis factor (TNF)-α,4 thus, inhibiting TNF-α induced pro-inflammatory cytokine expression.5 On the other hand, SIRT6 upregulates TNF-α protein synthesis in the presence of adequate Nicotinamide adenine dinucleotide (NAD+).6 Accordingly, modulation of SIRT1 or SIRT6 can lead to alteration of TNF-α activity.2
The current work aimed at evaluating the expression of SIRT1, SIRT6, TNF-α, and IFN-γ in both normal and psoriatic skin. This was done in an attempt to detect their possible dysregulation and inter-actions in the complex pathogenesis of psoriasis.
Patients and methods
This case-controlled study was carried out on 30 adult psoriatic patients and 22 healthy controls after approval of Dermatology Research Ethical Committee of the Faculty of Medicine, Cairo University and in conformation with the declaration of Helsinki (as revised in Tokyo 2004). Written informed consent was retrieved from all participants prior to commencement of the study. Pregnancy, lactation, autoimmune diseases, and/or other dermatologic diseases necessitated exclusion. Patients were off systemic treatment for at least 1 month and topical therapy for 2 weeks prior to inclusion. Complete history was obtained and clinical examination was performed with disease severity assessed using the Psoriasis Area and Severity Index (PASI).
Punch skin biopsies of 4 mm were retrieved from patients (lesional and peri-lesional) and controls. Biopsies were rinsed in ice-cold phosphate buffer saline (PBS), then homogenized in 300 uL PBS on ice. Ultra-sonication was performed followed by centrifugation for 15 min at 3000×g. The supernatant was separated for quantitative detection of tissue levels of SIRT1, SIRT 6, IFN-γ, and TNF-α by enzyme-linked immunosorbent assay (ELISA) (Sun Red Kits, Shanghai, PR China).
Comparison of numerical variables between study groups was done using the Student’s t test. Categorical data were compared using the Chi-square (χ2) test. Correlation between various variables was done using Pearson’s moment correlation equation.
Results
The current study was conducted on 30 patients with psoriasis and 22 controls. Their demographic and clinical data are illustrated in Table 1.
Table 1.
Demographic and clinical data of patients and controls.
| Variables | Patients (n = 30) | Controls (n = 22) | P value | ||
|---|---|---|---|---|---|
| Age (years) | Range | 19–75 | 18–72 | 0.171 | |
| Mean ± SD | 42.5 ± 14.4 | 37.0 ± 14.0 | |||
| Sex | Males | 18 (60%) | 14 (63.6%) | 0.79 | |
| Females | 12 (40%) | 8 (36.4%) | |||
| Disease duration (years) | Range: 1–45 Mean ± SD: 11.4 ±10.4 |
– | |||
| Extent (%) | Range: 5–70 Mean ± SD: 20 ± 14.0 |
– | |||
| PASI score | Range: 2.4–42.6 Mean ± SD: 10.1 ± 8.1 |
– | |||
| SIRT1 (ng/mL) Mean ± SD | Lesional | Peri-lesional | Controls | ||
| 10.39 ± 2.7742 | 14.070 ± 2.3938 | 16.251 ± 3.5122 | |||
| P value <0.001* | |||||
| P value <0.010* | |||||
| P value <0.001* | |||||
| SIRT6 (ng/mL) Mean ± SD | 4.949 ± 0.4290 | 4.267 ± 0.5439 | 3.618 ± 0.6128 | ||
| P value <0.001* | |||||
| TNF-α (ng/mL) Mean ± SD | 203.232 ± 29.1036 | 173.099 ± 23.1281 | 131.161 ± 11.9213 | ||
| P value <0.001* | |||||
| IFN-γ (pg/mL) Mean ± SD | 1013.703 ± 316.8799 | 649.267 ± 228.1068 | 375.454 ± 83.3938 | ||
| P value <0.001* | |||||
P value <0.05 is significant.
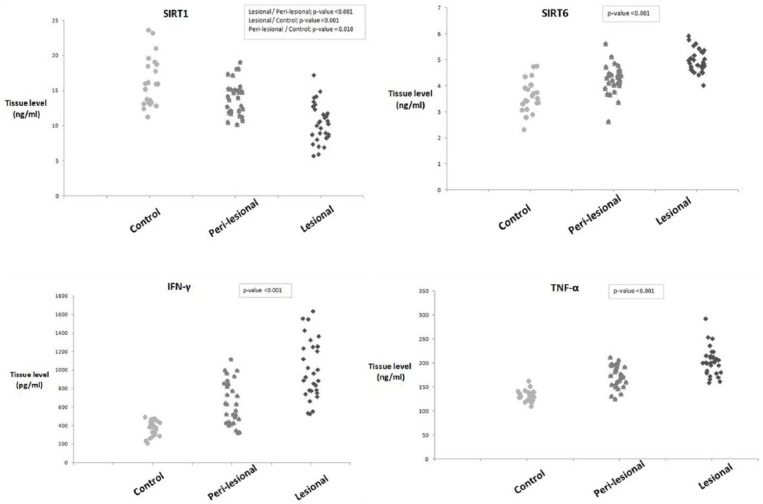
SIRT1 expression was lowest in psoriatic lesions with escalation of its tissue levels in peri-lesional skin to reach its highest value in controls (P <0.001), while SIRT6, TNF-α, and IFN-γ levels were significantly higher in lesional than peri-lesional skin and lowest in controls (P <0.001) (Table1) (Fig. 1).
Figure 1.
A graph showing tissue levels of SIRT1, SIRT6, IFN-γ, and TNF-α in lesional psoriatic skin, peri-lesional skin, and normal control skin. SIRT1 is significantly lower in psoriatic lesional and peri-lesional skin compared to controls, while SIRT6, IFN-γ, and TNF-α are significantly higher in psoriatic lesional and peri-lesional skin compared to controls.
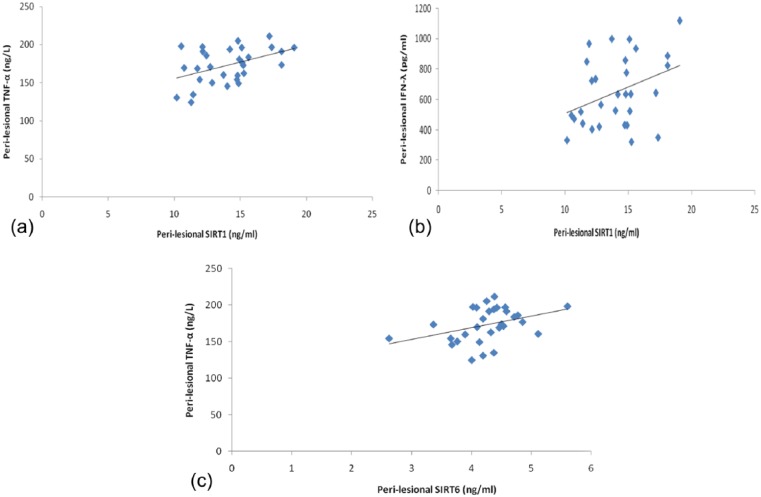
Disease duration showed a significant negative correlation with TNF-α in peri-lesional skin only (r = −0.483, P = 0.007). In peri-lesional skin, significant positive correlations were detected between SIRT1 and both TNF-α and IFN-γ (r = 0.451, P = 0.021 and r = 0.367, P = 0.046), respectively, and between SIRT6 and TNF-α (r = 0.377, P = 0.040) as well (Fig. 2). None of the other studied variables showed significant correlation with the demographic or clinical data of patients or controls.
Figure 2.
(a) Significant positive correlation between SIRT1 and TNF-α in peri-lesional skin of psoriatic patients. (b) Significant positive correlation between SIRT1 and IFN-γ in peri-lesional skin of psoriatic patients. (c) Significant positive correlation between SIRT6 and TNF-α in peri-lesional skin of psoriatic patients.
Discussion
In the current study, the possible involvement of two members of the SIRT family (SIRT1 and SIRT6) was highlighted adding to the complexity of the immune dysregulation associated with psoriasis. The expression of SIRT1 was detected to be significantly lowest in the lesional psoriatic skin followed by the peri-lesional areas and highest in the normal controls. On the other hand, the SIRT6 expression was found to be significantly increased in the psoriatic lesions compared to the peri-lesional skin and lowest in the normal controls. The expected upregulation of TNF-α and IFN-γ in psoriasis7 was further documented; however, their correlation with SIRT 1 and 6 in psoriatic skin could not be verified.
The possible role of SIRT1 in psoriasis has been suggested previously8,9 but with contradictory findings. Recently, the ability of SIRT1 activation to ameliorate imiquimod-induced psoriasiform phenotype and histology in mice has been demonstrated,10 positioning SIRT1 in the “good cup” side of psoriasis. In agreement with our results, Zhang et al.8 detected significantly reduced SIRT1 levels in psoriatic patients and attributed this to an intrinsic defect. In contrast, Sestito et al.9 documented no significant differences in SIRT1 levels measured in keratinocyte cultures of psoriatic and control skin. However, the latter authors reported that in skin samples, rather than in keratinocyte culture, basal keratinocytes’ SIRT1 expression was reduced in lesional psoriasis in comparison to healthy and non-lesional skin.
In the current study, surprisingly, a significant positive correlation between SIRT1 and both TNF-α and IFN-γ was detected in peri-lesional skin. This was unanticipated, as TNF-α and IFN-γ should allegedly downregulate SIRT18 and SIRT1 should suppress the pro-inflammatory cytokines.11 However, the notification that psoriatic keratinocytes with downregulated SIRT1 expression are able to upregulate and correct their SIRT1 status when cultured8 sheds light on the possible influence of the surrounding milieu on the behavior of keratinocytes, a postulation that can be elicited by in vitro experiments on keratinocytes cultures. Interestingly, Saini et al.12 reported that SIRT1 expression was elevated in myoblasts following incubation of TNF-α. The positive SIRT1 correlation with TNF-α and IFN-γ existence only in the peri-lesional skin could raise the speculation that SIRT1 is upregulated in a trial to keep the balance against the pro-inflammatory cytokines, a battle lost in the immunologically mature psoriatic lesional skin. Sirtuins important role in preserving immunological and inflammatory balances via different pathways has been recently highlighted.11 Examination of other cytokines involved in development of the psoriatic plaque in relation to SIRTs, particularly Tregs, may help clarify their intertwined relation. In addition, a recent study on SIRT1 activator in the treatment of patients with moderate to severe psoriasis revealed promising results with reasonable safety profile as mentioned by the authors.13 Such findings warrant further exploration of this field of new therapeutic options.
Regarding SIRT6, to our knowledge no other studies investigated its expression in psoriasis, with the current work being the first to draw attention to its possible involvement in such cases.
Several mechanisms have been proposed through which SIRT1 downregulation could be involved in psoriasis including inflammatory, immunological, and hyperproliferative pathways.14 Furthermore, SIRT6 upregulates TNF-α synthesis,6 with its deteriorating influences on psoriasis.7
The absence of a correlation between SIRT1 or SIRT6 and the studied clinical parameters (PASI or duration) does not subdue their roles, as psoriasis is a multifactorial disease,7 with many factors influencing its severity and chronicity. In the current study, we did not investigate the possible role of SIRT1 and SIRT6 on the development of metabolic syndrome that is known to be associated with psoriasis. This would have been of supplementary value, owing to their known influences on the cardiovascular system, glucogenesis and lipogenesis,15 the main pillars of the metabolic syndrome. Further work tackling this issue would add much in revealing the real impact of such molecules in psoriasis and its associations.
Sirtuins represent an emerging branch that could fill gaps in the complex pathogenesis of various diseases. This study adds strength to the advocated role of SIRT1 and points to SIRT6 as a possible culprit in psoriasis. SIRT1 activating and SIRT6 inhibitory drugs would offer a new therapeutic option for psoriasis.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Haigis MC, Sinclair DA. (2010) Mammalian sirtuins: Biological insights and disease relevance. Annual Review of Pathology 5: 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Serravallo M, Jagdeo J, Glick SA, et al. (2013) Sirtuins in dermatology: Applications for future research and therapeutics. Archives of Dermatological Research 305: 269–282. [DOI] [PubMed] [Google Scholar]
- 3. Kawahara TL, Chang HY, Chua KF, et al. (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeung F, Hoberg JE, Ramsey CS, et al. (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO Journal 23: 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu X, Liu Q, Wang M, et al. (2011) Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One 6: e27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Gool FV, Smedt TD, Alt FW, et al. (2009) Intracellular NAD levels regulate tumor necrosis factor protein synthesis in a sirtuin-dependent manner. Nature Medicine 15: 206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehlis SL, Gordon KB. (2003) The immunology of psoriasis and biologic immunotherapy. Journal of the American Academy of Dermatology 49: 44–50. [DOI] [PubMed] [Google Scholar]
- 8. Zhang P, Su Y, Zhao M, et al. (2011) Abnormal histone modifications in PBMCs from patients with psoriasis vulgaris. European Journal of Dermatology 21: 552–557. [DOI] [PubMed] [Google Scholar]
- 9. Sestito R, Madonna S, Scarponi C, et al. (2011) STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB Journal 25: 916–927. [DOI] [PubMed] [Google Scholar]
- 10. Xie S, Su Z, Zhang B, et al. (2015) SIRT1 activation ameliorates Aldara-induced psoriasiform phenotype and histology in mice. Journal of Investigative Dermatology 135(7): 1915–1918. [DOI] [PubMed] [Google Scholar]
- 11. Chen X, Lu Y, Zhang Z, et al. (2015) Intercellular interplay between Sirt1 signalling and cell metabolism in immune cell biology. Immunology 145(4): 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saini A, Al-Shanti N, Sharples AP, et al. (2012) Sirtuin 1 regulates skeletal myoblast survival and enhances differentiation in the presence of resveratrol. Experimental Physiology 97(3): 400–418. [DOI] [PubMed] [Google Scholar]
- 13. Krueger JG, Suárez-Fariñas M, Cueto I, et al. (2015) A randomized, placebo-controlled study of SRT2104, a SIRT1 activator, in patients with moderate to severe psoriasis. PLoS One 10(11): e0142081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blander G, Bhimavarapu A, Mammone T, et al. (2008) SIRT1 promotes differentiation of normal human keratinocytes. Journal of Investigative Dermatology 129(1): 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidovici BB, Sattar N, Prinz J, et al. (2010) Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. Journal of Investigative Dermatology 130: 1785–1796. [DOI] [PubMed] [Google Scholar]