Abstract
Mast cells are vital mediators of drug allergy and, therefore, studying the relationship between drug allergy and mast cells is essential. Sinomenine is the principal active component of Sinomenium acutum, which has anti-inflammatory and anti-immune effects, and is used to treat various rheumatoid diseases. However, allergic responses to sinomenine are frequently reported. Therefore, this study assessed the effects of sinomenine on mast cell activation to characterize its allergic effects and the underlying mechanisms. Enzyme-linked immunosorbent assay (ELISA), western blot analyses, and degranulation assays were performed to measure pro-inflammatory and allergic mediators in P815 cells. The allergenic effects of sinomenine were also determined in mice by using active general anaphylaxis (ASA). The results indicated that sinomenine induced inositol-1,4,5-trisphosphate (IP3) production and the release of histamine, interleukin (IL)-6, and endoplasmic reticulum Ca2+ in P815 cells. Furthermore, sinomenine upregulated the phosphorylation of sarcoma (Src), phospholipase C (PLC)-γ1, and IP3 receptor (R). Therefore, sinomenine induced concentration-dependent mast cell activation directly in vitro. Furthermore, our in vivo data identified an appropriate intravenous dose that did not induce these allergic effects, thereby providing information for the potential safe clinical use of sinomenine.
Keywords: anaphylaxis, degranulation, sinomenine
Introduction
Sinomenine (7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinane-6-one; Figure 1) is the principal active component of the plant Sinomenium acutum, which has anti-inflammatory and anti-immune properties that have been used successfully for centuries to treat patients with various rheumatoid diseases.1 It was recently reported to have anticancer,2,3 neuroprotective, and antidiabetic effects.4,5 Furthermore, pharmacological studies have demonstrated that sinomenine has significant immunosuppressive, anti-inflammatory, analgesic, and anti-arthritic properties.6,7 In addition, sinomenine was shown to inhibit the proliferation of fibroblast-like synoviocytes and production of pro-inflammatory cytokines in rheumatoid arthritis.6,7 These findings indicate that sinomenine has potential beneficial clinical effects for the treatment several diseases, including rheumatism. In addition, sinomenine may be suitable for long-term administration and significantly alleviate articular symptoms in patients with rheumatoid arthritis. An injectable sinomenine formulation has been developed and approved in China (Zheng Qing Feng Tong Ning injection, approval number Z43020279), and is currently widely used in patients.
Figure 1.
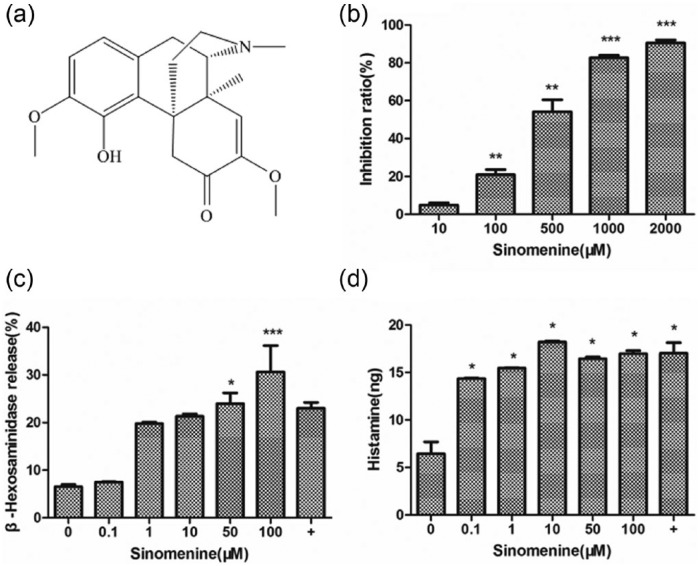
Structure and effects of sinomenine on P815 cell viability and degranulation. (a) Structure of sinomenine. (b) P815 cells were treated with indicated concentrations of sinomenine for 24 h prior to the 3-(4,5-dimethylthiazol-2-yl)-2,5-(diphenyltetrazolium) bromide (MTT) cell viability assay. Cells were incubated with indicated concentrations of sinomenine for 30 min prior to assay of (c) released β-hexosaminidase, expressed as a percentage of control, and (d) enzyme-linked immunosorbent assay (ELISA) of released histamine, expressed as fold change compared to control cells. Data are mean ± standard error of the mean (SEM) of three independent experiments; *P <0.05, **P <0.01, and ***P <0.001 vs. control cells (+, C48/80).
The widespread use of sinomenine has led to frequent reports of its allergenic effects.8 Most of these effects have manifested as anaphylactic reactions, and therefore, it is important to focus on this adverse effect of sinomenine administration. Anaphylaxis consists of a sequence of serious symptoms that constitute a clinical emergency and recent reports have suggested that it is increasing in prevalence.9 A central feature of anaphylaxis is the production of immunoglobulin E (IgE) as well as its interaction with receptors (mainly FcεRI) predominantly on mast cells, which are the key effectors that respond to allergens and release various mediators. These mediators include histamine, proteases (mainly tryptase), mast cell carboxypeptidase A3 (CPA3), chymase, platelet activating factor (PAF), prostaglandins (PGD2), leukotrienes (LTC4), and chemokines. Although mast cells are conventionally associated with IgE-mediated allergic responses and disease, there is accumulating evidence indicating that they are a crucial component of the innate immune system.10 Furthermore, anaphylaxis is one of the most widespread adverse events associated with the injection of traditional Chinese medicines.11,12
P815 cells are derived from the mouse mastocytoma cell line, which does not express the FcεR and, therefore, can be activated independent of the IgE pathway.10 Calcium ions are essential for MC/B activation and degranulation. PLCγ hydrolyzes phosphatidylinositol-4,5-bisphosphate to form soluble inositol-1,4,5-trisphosphate (IP3) and membrane-bound diacylglycerol (DAG). IP3 and DAG are second messengers that induce a series of complex modifications of mast cell physiology. The binding of IP3 to its receptor causes the “first wave” of calcium (Ca2+) mobilization, which is the transient release of Ca2+ from endoplasmic reticulum stores. This in turn induces a prolonged “second wave” of Ca2+ release through store-operated calcium entry. The massive entry of Ca2+ is necessary for the activation of the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) transcription factors, and both are crucial for the transcription of numerous cytokine genes, including IL-6, TNF-α, and IL-13.13,14
Diverse primary genetic and environmental factors may influence the susceptibility of an individual to anaphylactic reactions.15 The activation of mast cells or basophils initiates a series of biochemical events that result in the release of active mediators of the ensuing allergic reactions.12,16 The present study is focused on the anaphylactic effects of sinomenine, which were investigated using both in vitro and in vivo approaches.
Methods
Ethics statement and animals
This study was conducted in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH), Bethesda, MD, USA. The experimental protocols for using BALB/c mice were also approved by the Animal Ethics Committee of the Xi’an Jiaotong University (permit number: XJTU 2011-0045). Furthermore, 5–6-week-old male BALB/c mice were purchased from Xi’an Jiaotong University School of Medicine Laboratory Animal Center.
Cell culture
P815 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s minimal essential medium (Gibco, UK) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco, UK) and maintained in a 5% CO2 incubator at 37°C. When the cells had attained 80% confluence, they were digested, resuspended in fresh medium, and seeded in 96- or 6-well plates or 6-cm dishes for the experiments.
Cell viability assay
Exponentially growing cells (5.0 × 103 cells/well) were plated into 96-well plates (Coning, NY, USA). Twenty-four hours later, the cells were incubated in a medium containing different concentrations of sinomenine for 48 h at 37°C. Then, 20 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-(dipheny-ltetrazolium) bromide (MTT) solution (5 g/L) was added to each well and incubated for an additional 4 h at 37°C. The medium was discarded, and 150 μL of dimethyl sulfoxide was added to dissolve the formazan crystals that had formed. Then the absorbance of each well was examined at 490 nm by using a microplate reader (Bio-Rad, Hercules, CA, USA).
Measurements of cytokine and protease levels
The levels of interleukin (IL)-6), tumor necrosis factor (TNF)-α, and histamine were determined in conditioned cell culture media collected after incubation for 30 min with sinomenine, compound 48/80 (C48/80, a classical mast cell activator and canonical basic secretagogue, Sigma-Aldrich), or the vehicle using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers’ instructions.
β-hexosaminidase degranulation assay
Cells were harvested at the exponential growth phase, seeded into 96-well plates (2 × 104 cells/well), incubated at 37°C overnight, and then treated with different concentrations of sinomenine (25–200 μM) or C48/80 (30 μg/mL). The incubation was terminated after 30 min by placing the plates on ice for 10 min; the conditioned medium was collected, centrifuged at 1000 × g for 10 min at 4°C, and then the cells were lysed in assay buffer containing 1% (v/v) Triton X-100 (Xi ‘an Kehao Biological Engineering Co., Ltd., PR China). The hexosaminidase activity was subsequently determined by incubating either the conditioned medium or cell lysate with 1 mM β-hexosamine substrate for 90 min at 37°C. The reaction was terminated by adding 0.1 µM sodium carbonate (Na2CO3)/sodium bicarbonate (NaHCO3), and the absorbance was measured at 405 nm by using a microplate reader (Bio-Rad). The percentage of β-hexosaminidase released was calculated as follows: β-hexosaminidase release (%) = absorbance of conditioned medium/(absorbance of blank media + absorbance of blank cell lysate) × 100.
Ca2+ influx in P815 cells
P815 cells were loaded with 4 µM fluo-3 AM Ca2+ indicator for 20 min at 37°C in Hank’s balanced salt solution (HBSS) and incubated further for 40 min at 37°C after adding 5 volumes of HBSS containing 1% FBS. Then, the cells were washed thrice with HBSS, harvested, and centrifuged at 1000 rpm for 5 min at 4°C; the cell pellet was resuspended in HEPES, and then 5 × 104 cells/well were plated in 96-well plates. The cells were incubated in the presence or absence of sinomenine (0.1–100 µM) or C48/80 (30 µg/mL) for 1 min and fluorescence was measured using a microplate fluorimeter at excitation and emission wavelengths of 488 and 527 nm, respectively.
Active general anaphylaxis in mice
Mice were injected with C48/80 (2.5 mg/kg), sinomenine (0.364, 1.82, or 9.10 mg/kg), or the vehicle intravenously through the caudal vein combined with 0.4% Evans blue (n = 8 mice/group). The animals were euthanized 30 min after this challenge, and their ears were removed and processed to measure the dye extravasation after the dye had been extracted overnight from the ear with 800 μL of a mixture of acetone-saline (7:3) at 65°C. The absorbance was measured at 620 nm.
IP3 and TNF-α levels in mouse serum
Blood samples were collected from mice that were administered C48/80 (2.5 mg/kg), sinomenine (0.364, 1.82, or 9.10 mg/kg), or the vehicle intravenously via the caudal vein. The serum was obtained by allowing the blood to stand at room temperature for 30 min, followed by centrifugation for 10 min (1000 × g). Serum IP3 and TNF-α levels were determined using ELISA kits following the manufacturers’ instructions.
Western blot analysis
P815 cells were exposed to different concentrations of sinomenine, C48/80, or the vehicle for 30 min, and then lysed on ice with radioimmunoprecipitation assay (RIPA) lysis buffer supplemented with 15 mL of protease (Roche) and phosphatase inhibitor cocktails. The western blot analysis was performed using a previously described method.17 The cell lysates were analyzed using primary antibodies against phosphorylated (p)-Src, Lyn, IP3R, p-IP3R, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), followed by detection using enhanced chemiluminescence. The protein band densities were analyzed using the Quantity One 1-D analysis software (version 4.4, Bio-Rad).
Statistical analyses
The data are presented as the mean ± standard error of mean (SEM) and were statistically analyzed using analysis of variance (ANOVA). Two-tailed tests were used for two-group comparisons and differences were considered statistically significant at P <0.05.
Results
Sinomenine induced degranulation in P815 cells
Following activation, mast cells release preformed granule-stored mediators such as histamine and β-hexosaminidase, which are known markers of this cell type. Therefore, we assess the effect of various doses of sinomenine on degranulation by evaluating the release of these two granule markers in P815 cells. β-hexosaminidase release significantly increased in a concentration-dependent manner in cells exposed to 0.1, 1, 10, 50, and 100 μM sinomenine (Figure 1c). In addition, sinomenine induced marked histamine release at a low concentration (0.1 μM), although this effect tended to decrease as the sinomenine concentration increased (Figure 1d). These results indicate that sinomenine induced cell degranulation in vitro.
Sinomenine increased vessel permeability in mice
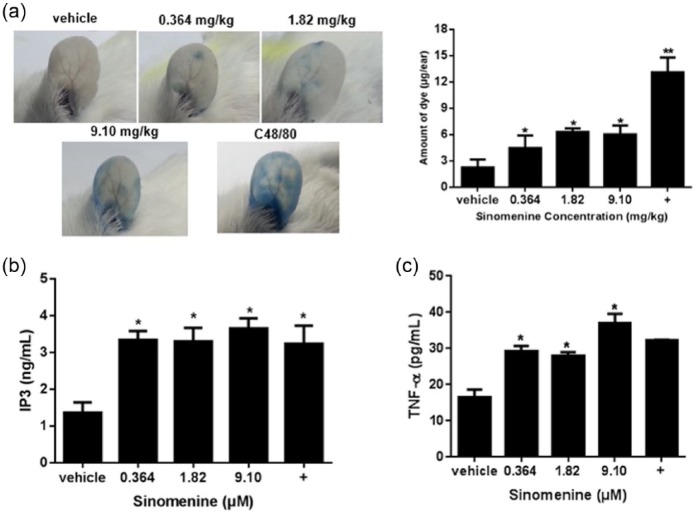
We investigated the allergenic effects of sinomenine in vivo by injecting it or C48/80 intravenously into the mice via the caudal vein with or without 4% Evans blue dye. Treatment with sinomenine increased the dye diffusion by 50%, suggesting that sinomenine increased ear vessel permeability (Figure 2a). ELISA analysis of the mouse serum showed that sinomenine increased IP3 and TNF-α release at a low dose (0.364 mg/kg) in treated mice. When the dose was increased, the release of TNF-α and IP3 was sustained at high levels. Taken together, these results indicate that sinomenine may induce an allergic response.
Figure 2.
Allergenic effect of sinomenine in mice. Mice received indicated doses of sinomenine, C48/80 (+), or vehicle (a) plus Evans blue dye (0.4%) intravenously via caudal vein and ear images were acquired 1 h later and (b) followed by determination of serum inositol-1,4,5-trisphosphate (IP3) and tumor necrosis factor (TNF)-α levels; *P <0.05 and **P <0.01 vs. control group.
Sinomenine enhanced IP3 production and intracellular Ca2+ levels in P815 cells
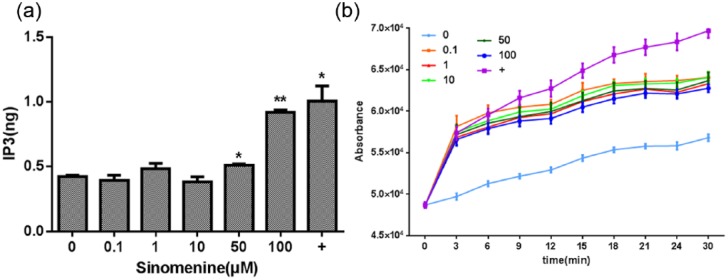
Massive IP3-induced Ca2+ entry is necessary for the activation of the NF-κB and NFAT transcription factors, which are both crucial for the transcription of numerous cytokine genes, including IL-6, TNF-α, and IL-13. Furthermore, enhanced intracellular Ca2+ levels promote degranulation. Therefore, we determined the effects of sinomenine on IP3 and Ca2+, and our data (Figure 3) revealed that it induced significant Ca2+ mobilization at high concentrations (50 or 100 μM). The highest concentration of sinomenine (100 μM) also enhanced IP3 production by 91.65 ± 1.21% compared with the control cells.
Figure 3.
Effect of sinomenine on inositol-1,4,5-trisphosphate (IP3) release and Ca2+ mobilization in P815 cells. (a) Cells were treated with indicated concentrations of sinomenine for 30 min, and IP3 release was measured using enzyme-linked immunosorbent assay (ELISA) and expressed as fold change compared to control cells. (b) Cells were treated with indicated concentrations of sinomenine, vehicle, or C48/80 (+) for 1 min prior to intracellular Ca2+ measurement using fluo-3 AM. Data are expressed as change in fluo-3 AM fluorescence; *P <0.05 and **P <0.01 vs. control cells.
Effect of sinomenine on P815 cell signaling events
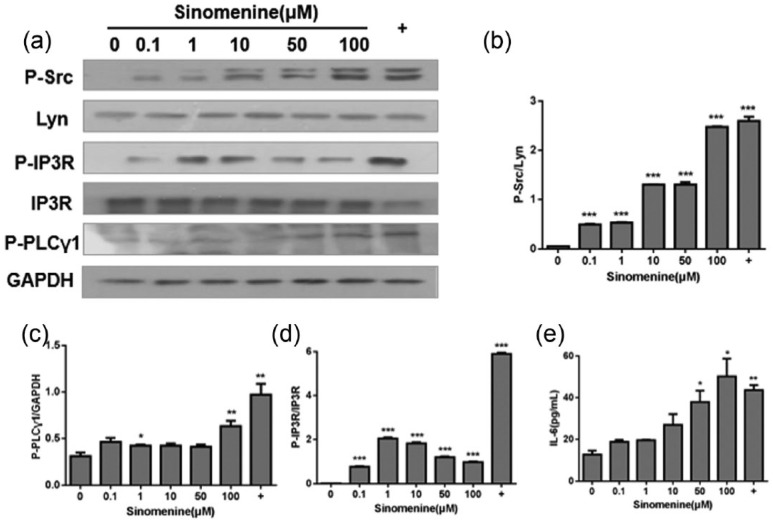
IL-6 is a cytokine that plays an important role in allergic diseases and the IP3R is a key regulator of Ca2+-independent degranulation. IP3R is the receptor of IP3 which activated by PLCγ, when IP3 binding to its receptor, Ca2+ released from endoplasmic reticulum and cell degranulation occurred, Lyn also activates Syk tyrosine kinase and several adaptor proteins. Syk in turn phosphorylates many signaling proteins resulting in a reduced capacity to degranulate and to generate cytokines. We investigated the effect of sinomenine on relevant signaling pathway molecules in P815 cells and found that concentration-dependently increased the IP3R protein level (Figure 4d) and altered the levels of PLCγ and p-Src (Figure 4b and c). Furthermore, sinomenine upregulated the protein levels of p-Lyn and PLCγ1 as well.
Figure 4.
Effect of sinomenine on Lck-Yes novel tyrosine kinase/phospholipase C-γ/inositol 1,4,5, triphosphate receptor (Lyn/PLCγ/IP3R) pathway in P815 cells Cells were treated with indicated concentrations of sinomenine for 30 min. (a) Western blot bands of target proteins. Levels of (b–d) phosphorylated (p)-Src, PLCγ, and IP3R after treatment with sinomenine and (e) secreted interleukin (IL)-6. Data are mean ± standard error of the mean (SEM) of three independent experiments; *P <0.05, **P <0.01, and ***P <0.001 vs. control cells.
Discussion
Mast cells are the primary effectors of allergic reactions and, therefore, have critical roles in the development of allergenic diseases. These cells secrete histamine as well as various inflammatory and immunomodulatory substances that are produced during anaphylactic reactions. The present study confirmed the in vitro and in vivo allergic effects of sinomenine in P815 mast cells. When IgE binds to its FcεRI receptor on mast cells, aggregation occurs, which activates Lyn and then Syk tyrosine kinase is subsequently phosphorylated, leading to mast cell activation.12,18,19 For our in vitro study, we utilized P815 mast cells to determine the effect of sinomenine on non-FcεR-mediated activation of mast cells because these cells do not express the FcεR.10 We showed that sinomenine directly induced cell degranulation and release of pro-inflammatory mediators including β-hexosaminidase and histamine, concentration-dependently. Furthermore, the western blot analysis showed that sinomenine upregulated the phosphorylation of Ca2+ influx-related proteins, including Src, PLCγ, and IP3R. Therefore, the sinomenine-induced mast cell activation was initiated by Ca2+ upregulation. Following IgE-mediated mast cell activation, phosphorylated Syk further phosphorylates numerous signaling proteins, which are required for assembling the membrane-localized signaling networks. This includes PLCγ, which hydrolyzes phosphatidylinositol-4,5-bisphosphate to form soluble IP3 and membrane-bound DAG, which are both second messengers that induce a series of complex modifications to mast cell physiology.
The binding of IP3 to its receptor causes the “first wave” of transient Ca2+ release from endoplasmic reticulum stores, which subsequently induces a prolonged “second wave” of Ca2+ from the endoplasmic reticulum as well as an influx via calcium release-activated calcium modulator 1 (ORAI-1) on the plasma membrane.16,20,21 This Ca2+ influx subsequently produces eicosanoids and lipid mediators.22,23 In addition to the IgE-mediated route, mast cells can be activated by exposure to the basic secretagogue, C48/80,24 which we used in our in vivo investigations to confirm the allergic effect of sinomenine in mice, where it enhanced blood vessel permeability and dose-dependently increased in serum IP3 and TNF-α levels.
In conclusion, these findings demonstrated that sinomenine was directly bound to the mast cell membrane and activated Lyn and Syk, which triggered the PLCγ-mediated IP3 production. Furthermore, cytoplasmic free IP3 bound to the endoplasmic reticulum IP3R, triggering Ca2+ release, which resulted in mast cell degranulation and the release of the anaphylactic mediators, β-hexosaminidase and histamine. Therefore, our study provided evidence of the allergenic effects of sinomenine, identified an appropriate intravenous dose that did not induce allergic effects, and demonstrated the possible underlying mechanisms mediating these actions in P815 mast cells, thereby providing information for the potential safe clinical use of sinomenine.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported by grants from the National Natural Science Foundation of China (nos. 81503321 and 81230079 and 81227802) and the National “Twelfth Five-Year” Plan for Science & Technology Support of China (no. 2012BAI29B06).
References
- 1. Zhao XX, Peng C, Zhang H, et al. (2012) Sinomenium acutum: A review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharmaceutical Biology 50: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 2. Li X, Wang K, Ren Y, et al. (2014) MAPK signaling mediates sinomenine hydrochloride-induced human breast cancer cell death via both reactive oxygen species-dependent and -independent pathways: An in vitro and in vivo study. Cell Death & Disease 5: e1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Z, Duan ZJ, Chang JY, et al. (2014) Sinomenine sensitizes multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by downregulation of MDR-1 expression. PLoS One 9: e98560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Yang Z, Liu YP, Yuan F, et al. (2014) Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Molecular Immunology 60: 109–114. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Yang F, Xiang Y, et al. (2014) Preventive effects of sinomenine on the development of diabetes in NOD mice. Latin American Journal of Pharmacology 33: 87–92. [Google Scholar]
- 6. Jiang Y, Gao M, Wang WM, et al. (2015) Sinomenine hydrochloride protects against polymicrobial sepsis via autophagy. International Journal of Molecular Science 16: 2559–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang HM, Ren Y, Tang XJ, et al. (2015) Vascular normalization induced by sinomenine hydrochloride results in suppressed mammary tumor growth and metastasis. Scientific Reports 5: 8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ge HX, Li Q, Lei ZB. (2010) Adverse reaction and rational drug use advice of Zheng Qing Feng Tong Ning. Chinese Traditional Patent Medicine 32: 287–289. [Google Scholar]
- 9. Nwaru BI, Sheikh A. (2015) Anaphylaxis in adolescents: A potential tripartite management framework. Current Opinion in Allergy and Clinical Immunology 15: 344–349. [DOI] [PubMed] [Google Scholar]
- 10. Thathiah P, Sanapala S, Rodriguez AR, et al. (2011) Non-Fc epsilon R bearing mast cells secrete sufficient interleukin-4 to control Francisella tularensis replication within macrophages. Cytokine 55: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hou SX, Yuan LF, Du F, et al. (2012) Investigation and analysis on the adverse reactions caused by 12 kinds of commonly used traditional Chinese medicine injections. African Journal of Microbiology Research 6: 2797–2801. [Google Scholar]
- 12. Lee JK, Vadas P. (2011) Anaphylaxis: Mechanisms and management. Clinical and Experimental Allergy 41: 923–938. [DOI] [PubMed] [Google Scholar]
- 13. Olenchock BA, Guo R, Silverman MA, et al. (2006) Impaired degranulation but enhanced cytokine production after Fc epsilon RI stimulation of diacylglycerol kinase xi-deficient mast cells. Journal of Experimental Medicine 203: 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sibilano R, Frossi B, Pucillo CE. (2014) Mast cell activation: A complex interplay of positive and negative signaling pathways. European Journal of Immunology 44: 2558–2566. [DOI] [PubMed] [Google Scholar]
- 15. Ben-Shoshan M, Clarke AE. (2011) Anaphylaxis: Past, present and future. Allergy 66: 1–14. [DOI] [PubMed] [Google Scholar]
- 16. Metcalfe DD, Peavy RD, Gilfillan AM. (2009) Mechanisms of mast cell signaling in anaphylaxis. Journal of Allergy and Clinical Immunology 124: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang N, Zhang Y, Wu L, et al. (2014) Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology 79: 282–289. [DOI] [PubMed] [Google Scholar]
- 18. Lee YS, Hur S, Kim TY. (2014) Homoisoflavanone prevents mast cell activation and allergic responses by inhibition of Syk signaling pathway. Allergy 69: 453–462. [DOI] [PubMed] [Google Scholar]
- 19. Joo HM, Kang SJ, Nam SY, et al. (2015) The Inhibitory Effects of Low-Dose Ionizing Radiation in IgE-Mediated Allergic Responses. Plos One 10: e0136394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo Y, Han SL, Cao JJ, et al. (2014) Screening of allergic components mediated by H1R in homoharringtonine injection through H1R/CMC-HPLC/MS. Biomedical Chromatography 28: 1607–1614. [DOI] [PubMed] [Google Scholar]
- 21. Ashmole I, Duffy SM, Leyland ML, et al. (2012) CRACM/Orai ion channel expression and function in human lung mast cells. Journal of Allergy and Clinical Immunology 129: 1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kambayashi T, Koretzky GA. (2007) Proximal signaling events in Fc epsilon RI-mediated mast cell activation. Journal of Allergy and Clinical Immunology 119: 544–552. [DOI] [PubMed] [Google Scholar]
- 23. Zhang T, Han SL, Liu Q, et al. (2014) Analysis of allergens in tubeimu saponin extracts by using rat basophilic leukemia 2H3 cell-based affinity chromatography coupled to liquid chromatography and mass spectrometry. Journal of Separation Science 37: 3384–3391. [DOI] [PubMed] [Google Scholar]
- 24. Yao JH, Cui M, Li MT, et al. (2014) Angiopoietin1 inhibits mast cell activation and protects against anaphylaxis. PLoS One 9: e89148. [DOI] [PMC free article] [PubMed] [Google Scholar]