Dear Editor,
In their recent work, Molinaro et al. discussed all the possible causes of intervening infections in patients affected by membranous nephropathy (MN).1 Among these recognized factors, one of the most relevant is represented by the immunosuppressive regimen to which these patients are subjected. Moreover, they proposed the possible employment of immunoglobulins injection as prophylactic measure, to avoid the development of an infectious disease in patients already compromised. These kinds of therapies should be evaluated along with the classical immunodepleting drugs routinely used to treat this nephropathy. For this reason, a correct diagnostic assessment of these cases is crucial, and the performance of renal biopsy is mandatory to identify and characterize the renal disease of the patient. In this direction, recently many studies have focused on the mechanism implicated in the development of a particular form of MN, the idiopathic one, finding interesting clues mainly regarding circulating auto-antibodies directed against normally expressed antigens on the membrane of podocytes (e.g. PLA2R or THSD7A).2,3 This information may have a strong impact both on the diagnostic and therapeutic approach to the patient with MN, conditioning the choice of the most appropriate treatment for each case.4–8 In fact, the employment of immunodepleting treatments may lead to the exacerbation of an underlying infection or the onset of a new one. In our recent experience, a 40-year-old woman, with a 6-year diagnosis of MN (Figure 1a and 1b) presented to the Nephrology Department referring oliguria, increased serum creatinine (5.8 mg/dl), nephrotic range proteinuria (8 g/day), and pretibial edema. She was treated with various immunosuppressive agents (steroid drugs and cyclophosphamide) for frequent relapses of the nephrotic syndrome over the years; however, the patient underwent a further renal biopsy to assess the cause of the unusual overlap of relapsing nephrotic syndrome and acute renal failure. The light microscopy analysis of the specimen showed that all the glomerular structures were affected by marked endo-capillary and extra-capillary proliferation, with the formation of prominent cellular crescents and exudative aspects (Figure 1c), associated with segmental thickening of the capillary loop basement membranes (Figure 1d). The immunofluorescence resulted positive to IgG, C3, and C1q fragments antisera. The electron microscopy assay highlighted the presence of the classic subepithelial immunodeposits of the MN, associated with mesangial and sub-endothelial immunocomplexes, without clear image compatible with humps (Figure 1e and 1f). These findings were strongly suggestive for a proliferative/crescentic form of glomerulonephritis, in differential diagnosis among an ANCA-associated vasculitis, an acute post-infectious glomerulonephritis, and a flare of lupus nephritis. After an exhaustive clinical investigation, the patient resulted negative to anti-ANCA antibodies in serum sample and did not show any other vasculitis manifestations. Moreover, the absence of previous infections was inconsistent with the diagnosis of acute post-infectious GN. Finally, even though the acute onset of the disease, the coexistence of proliferative and membranous alterations of the glomerular structures, and the positivity to C1q antisera at immunofluorescence analysis were suggestive for an atypical form of lupus nephritis, the absence of other EULAR criteria, the long renal disease history and the lack of the classic “full-house” immunofluorescence pattern were inconsistent with this diagnosis. Moreover, an immunohistochemistry (IHC) with antibodies directed against PLA2R and IgG4 antigens was performed, showing a positive, dot-like pattern, mainly in those areas spared from proliferative/exudative changes (Figure 1g and 1h). This finding, along with a PLA2R antibodies serum titer of 74.5 RU/mL, was suggestive for an idiopathic MN with superimposed acute crescentic damage. For all these reasons, to control the progressive acute renal injury, a further immunosuppressive therapy was administered to the patient, with slight improvement of the clinical condition. After 1 week of therapy, probably due to the massive immunodepleting regimen, the woman developed fever and fatigue, undergoing blood analysis and cultures with the isolation of Streptococcus spp. in serum samples. Moreover, a cardiac ultrasound showed the presence of a vegetation on the mitral valve, allowing the clinicians to diagnose a bacterial endocarditis. This information led us to adequately interpret the lesions previously noted on renal biopsy. Actually, the proliferative/crescentic changes in glomerular structures may be considered as the early manifestation of a latent bacterial endocarditis, representing a classical form of endocarditis-associated glomerulonephritis.9–14 The induction of opportunistic infections in MN-treated patients was described in the literature,15,16 with only exceptional cases of MN therapy-induced endocarditis. However, no previous case showed an exacerbation of the renal disease,17 so we would stress the importance of an adequate correlation between the histopathological and clinical data to avoid iatrogenic effects of inappropriate regimens, as confirmed in the paper by Molinaro et al.1 In these cases, of course, the priority is the clinical stabilization of the patient and the resolution of the progressing acute kidney failure. However, the institution of a wrong therapy could exacerbate the condition, producing a contrary effect. For this reason, the correct definition of the diagnosis is crucial in order to allow the choice of the most appropriate therapeutic approach (in this case, an antibiotic regimen or, as proposed by authors, immunoglobulins), and this point can be achieved only through an accurate clinic-pathological correlation. Finally, in the future, the introduction of new target therapies to treat these cases may allow to spare an immunodeficiency state to those patients affected by membranous nephropathy, avoiding the development of overlapped post-infectious nephritis.
Figure 1.
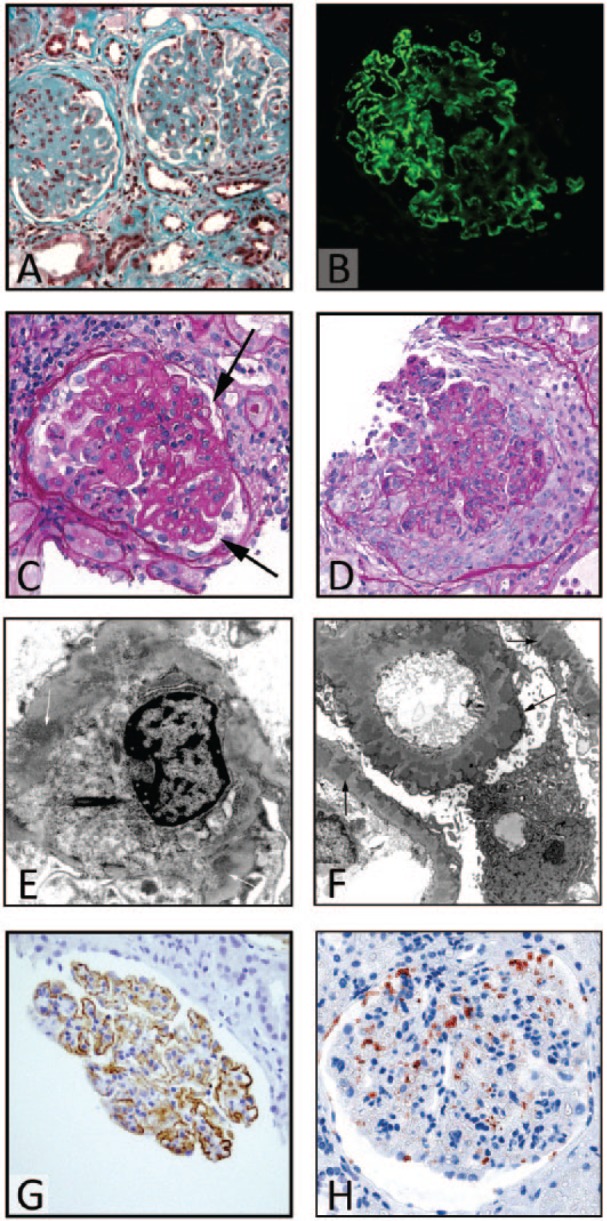
(a) A wide thickening of the glomerular basement membranes (GBMs) with a prominent sclerosis of the glomerular tuft (trichrome Masson stain, magnification ×20). (b) The positivity of the first biopsy to IgG antiserum at an immunofluorescence analysis (magnification ×20). (c) The second biopsy shows a residual aspect of underlying iMN, with the characteristic thickening of GBMs (arrow) (Periodic acid of Schiff, PAS stain, magnification ×20). In the same biopsy, the other glomeruli presented global endo/extra-capillary proliferative features, with a semi-circumferential cellular crescent formation ((d) PAS stain, magnification ×20). The electron microscopy (e, f) showed the presence of mesangial (white arrows) and sub-endothelial (black arrows) immunocomplexes, without clear images of humps structures. Finally, the IHC directed against PLA2R and IgG4 (g, h), respectively, showed a typical, dot-like, coarse granular positivity, especially in those regions with a preserved glomerular architecture, further confirming the diagnosis of underlying idiopathic membranous nephropathy (magnification ×20).
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Molinaro I, Barbano B, Rosato E, et al. (2014) Safety and infectious prophylaxis of intravenous immunoglobulin in elderly patients with membranous nephropathy. International Journal of Immunopathology and Pharmacology 27: 305–308. [DOI] [PubMed] [Google Scholar]
- 2. Beck LH, Jr, Bonegio RG, Lambeau G, et al. (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. New England Journal of Medicine 361: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, et al. (2014) Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. New England Journal of Medicine 371: 2277–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ponticelli C, Zucchelli P, Passerini P, et al. (1992) Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. The Italian Idiopathic Membranous Nephropathy Treatment Study Group. New England Journal of Medicine 327: 599–603. [DOI] [PubMed] [Google Scholar]
- 5. Ponticelli C, Zucchelli P, Passerini P, et al. (1989) A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. New England Journal of Medicine 320: 8–13. [DOI] [PubMed] [Google Scholar]
- 6. Ponticelli C, Zucchelli P, Imbasciati E, et al. (1984) Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. New England Journal of Medicine 310: 946–950. [DOI] [PubMed] [Google Scholar]
- 7. Ponticelli C, Zucchelli P, Passerini P, et al. (1995) A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney International 48: 1600–1604. [DOI] [PubMed] [Google Scholar]
- 8. Ramachandran R, Hn HK, Kumar V, et al. (2016) Tacrolimus combined with corticosteroids versus Modified Ponticelli regimen in treatment of idiopathic membranous nephropathy: Randomized control trial. Nephrology (Carlton, Vic) 21: 139–146. [DOI] [PubMed] [Google Scholar]
- 9. Balafa O, Kalaitzidis R, Liapis G, et al. (2015) Crescentic glomerulonephritis and membranous nephropathy: a rare coexistence. International Urology and Nephrology 47: 1373–1377. [DOI] [PubMed] [Google Scholar]
- 10. Thajudeen B, John SG, Ossai NO, et al. (2014) Membranous nephropathy with crescents in a patient with Hashimoto’s thyroiditis: A case report. Medicine 93: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu ZJ, Niu K, Liu B, et al. (2014) A case of membranous nephropathy and myeloperoxidase anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Experimental and Therapeutic Medicine 8: 1170–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez EF, Nasr SH, Larsen CP, et al. (2014) Membranous nephropathy with crescents: a series of 19 cases. American Journal of Kidney Diseases 64: 66–73. [DOI] [PubMed] [Google Scholar]
- 13. Nayak SG, Satish R. (2007) Crescentic transformation in primary membranous glomerulopathy: association with anti-GBM antibody. Saudi Journal of Kidney Diseases and Transplantation 18: 599–602. [PubMed] [Google Scholar]
- 14. Kanodia K, Vanikar A, Patel R, et al. Membranous nephropathy with MPO-ANCA-associated crescentic GN. Nephro-Urology Monthly 6: e20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grahammer F, Fischer KG. (2015) Pulmonary infiltrate and painful nodular leg lesions in a patient with membranous glomerulonephritis. BMJ Case Reports DOI: 10.1136/bcr-2015-210032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. López-Lluva MT, de la Nieta-Garcia MD, Pigueras-Flores J, et al. (2014) Chlorambucil-induced cytomegalovirus infection: a case report. Journal of Medical Case Reports 8: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iida H, Mizumura Y, Uraoka T, et al. (1985) Membranous glomerulonephritis associated with enterococcal endocarditis. Nephron 40: 88–90. [DOI] [PubMed] [Google Scholar]


