Figure 8.
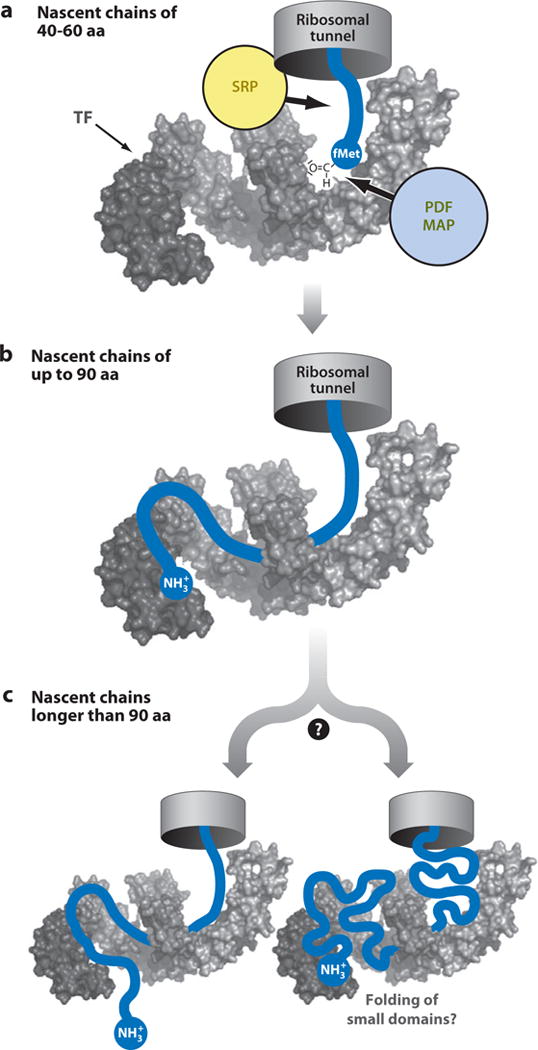
Mode for TF chaperone binding to nascent polypeptides based on cross-linking experiments by Merz et al. (59). TF directs the nascent chains through its interior in a sequence- and length-dependent manner. Interactions with TF are (a) moderate for nascent chains 40 to 60 residues long, and (b) considerable for nascent chains up to 90 residues, where the nascent chain’s N terminus reaches up to the TF PPIase domain (head). (c) Upon further elongation, the nascent chain may leave TF or it may accumulate in the interior of the TF chaperone. Abbreviations: PDF, protein deformylase; MAP, methionine aminopeptidase; SRP, signal recognition particle; TF, trigger factor. Adapted by permission from Macmillan Publishers Ltd: EMBO Journal (Reference 59), copyright (2008).
