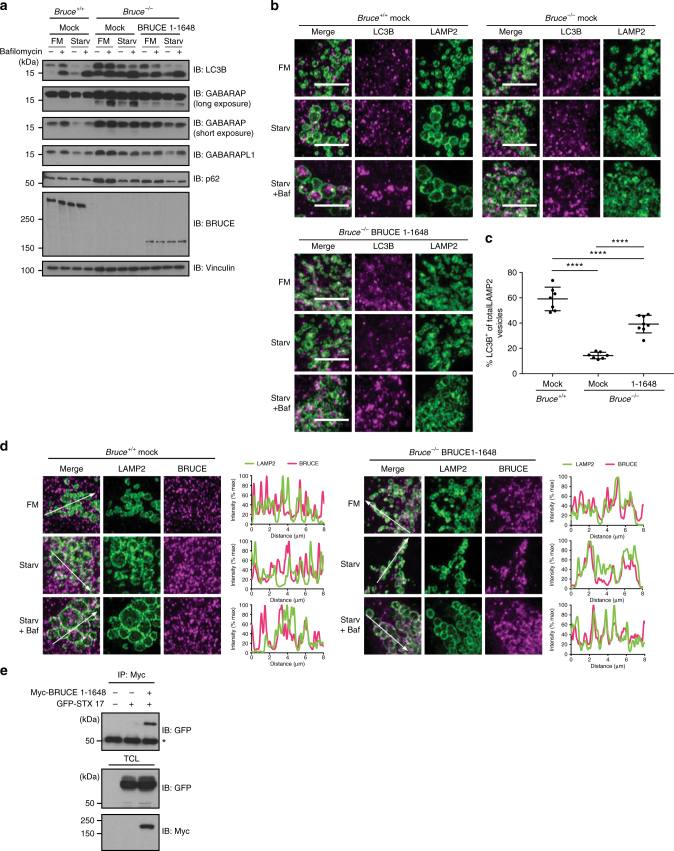
Fig. 7.
BRUCE (aa 1–1648) partially rescues the autophagy defect in Bruce−/− MEFs. a Protein levels of LC3B, GABARAP, GABARAPL1, and p62 in Bruce−/− MEFs stably expressing BRUCE 1–1648 compared with Bruce+/+ and Bruce−/− empty vector (mock) expressing MEFs. Total cell lysate of MEFs in fully supplemented medium (FM), starved for 2 h (Starv) with or without Bafilomycin A1 (Baf; 100 nM) was analyzed by immunoblotting using antibodies as indicated. Vinculin was monitored as a loading control. b Confocal microscopy images of LC3B and LAMP2 in Bruce+/+ (mock) and Bruce−/− (mock and BRUCE 1–1648) MEFs. MEFs in basal or 4 h starved condition with or without Bafilomycin A1 (100 nM) were fixed and stained for endogenous LC3B and LAMP2 using antibodies as indicated. Scale bars, 5 µm. c Quantification of LAMP2-positive vesicles containing LC3B-positive aggregates based on microscopy images from (b) in 4 h starved condition treated with Bafilomycin A1. Data are presented as dot plots with mean±SD (****p < 0.0001). n = 8 cells. d Colocalization analysis of LAMP2 and BRUCE in Bruce+/+ and Bruce−/− mock compared to BRUCE 1–1648 expressing MEFs. MEFs in basal, 4 h-starved condition with or without Bafilomycin A1 (100 nM) were fixed and stained for endogenous BRUCE and LAMP2 using antibodies as indicated. Fluorescent intensity of LAMP2 and BRUCE signals across 8 µm regions marked with arrows is shown in line plots. e Interaction of GFP-STX17 and Myc-BRUCE (aa 1–1648) determined by co-immunoprecipitation. GFP-STX17 and Myc-BRUCE (aa 1–1648) were transfected in HEK293T cells. Myc-BRUCE (aa 1–1648) was immunoprecipitated using anti-Myc antibody from total cell lysates (TCL). Immunoprecipitated samples were examined by immunoblotting using the indicated antibodies. *Nonspecific band