Figure 1.
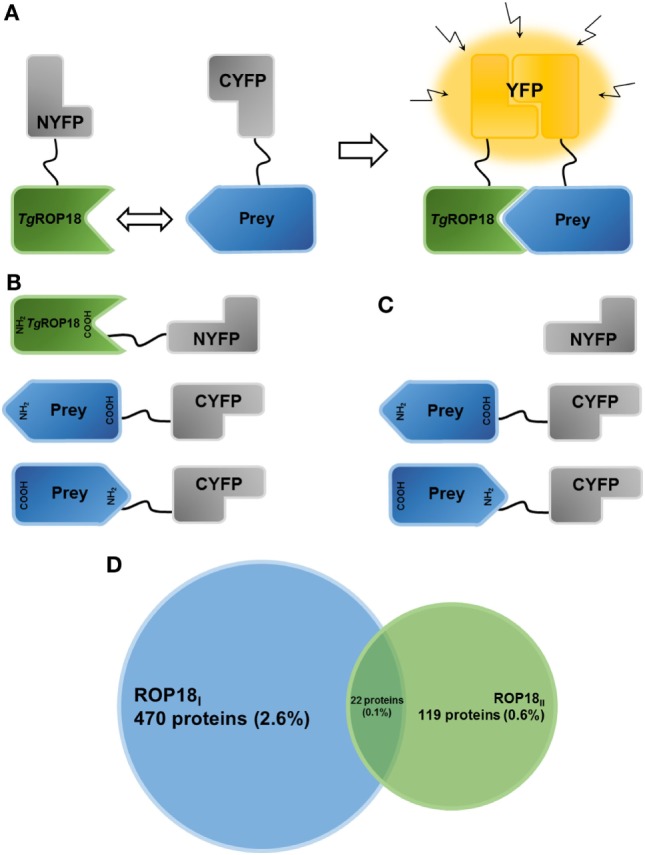
Establishment of the HT-BiFC screening system and TgROP18-interacting proteins. (A) Principle of the bimolecular fluorescence complementation (BiFC) assay. The non-fluorescent fragments of a fluorescent reporter protein are fused with the proteins of interest and expressed in human cells. If the interaction between the proteins of interest takes place, the split fragments will be pulled close enough to refold together and reconstitute the functional fluorescent entity. (B) Schematic representation illustrating the TgROP18/prey BiFC constructs generated in the present study. TgROP18 is fused with the N-terminal fragment of YFP (NYFP) at the C-terminus, and the prey protein is tethered with the C-terminal fragment of YFP (CYFP) at either the N- or C-terminus. (C) Schematic representation illustrating the control screening. Non-fused NYFP is mated with each CYFP-prey/prey-CYFP constructs. (D) Venn diagram depicting the number (percentage) of ROP18I-specific targets (blue), ROP18II-specific targets (green), and ROP18I/ROP18II targets (in the middle).
