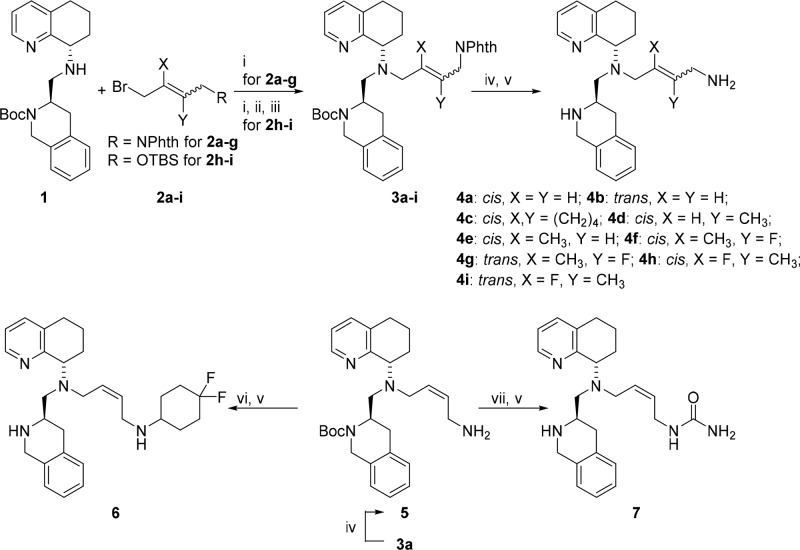
Scheme 1. Synthesis of Compounds 4a–i, 6, and 7.
Reagents and conditions: (i) 0.1 equiv of KI, 1.5 equiv of Et(iPr)2N, acetonitrile, 60–78%; (ii) 1.5 equiv of 1 M n-Bu4N+F– (THF), 53%; (iii) 1.2 equiv of phthalimide, 1.15 equiv of PPh3, 1.2 equiv of DIAD, THF, 78–100%; (iv) 8 equiv of 24 wt % N2H4 in water, MeOH, quant.; (v) 30 equiv of CF3COOH, CH2Cl2, 43–100%; (vi) 1.3 equiv of 4,4-difluorocyclohexanone, 1.2 equiv of CH3COOH, 1.5 equiv of NaBH(OAc)3, DCE, 81%; (vii) 1.3 equiv of 85 wt % (CH3)3SiNCO, 2 equiv of Et(iPr)2N, THF, 86%.