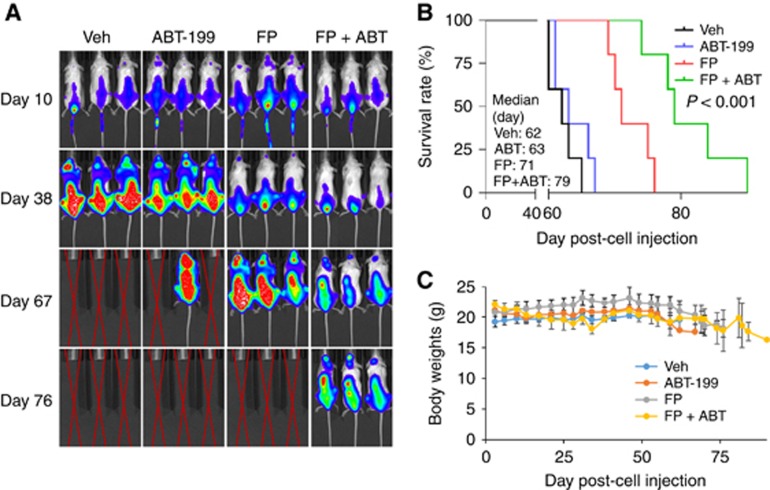
Figure 6.
The FP/ABT-199 regimen is active in an intravenous BM-homing murine model. (A) NOD/SCID-γ mice were injected intravenously via tail vein with 5 × 106 PS-R cells stably expressing luciferase. After signals were visible (for example, 10 days after injection of tumour cells), FP (3 mg kg−1, i.p.)±ABT-199 (100 mg kg−1, p.o.) was administered daily for the first 7 days, followed by FP (5 mg kg−1, i.p)±ABT-199 (100 mg kg−1, p.o.) 5 days a week for an additional 4 weeks; n=5 per group. Control animals were administered equal volumes of vehicle. Tumour cells were monitored every other day after i.p. injection with 150 mg kg−1 luciferin using the IVIS 200 imaging system. Veh, vehicle; (B) Survival of the animals was determined by Kaplan–Meier analysis. Inset, median survival days. P-value indicates significant difference between groups; (C) Mice did not display significant body weight loss (⩾20% of initial weight) or other signs of toxicity due to the treatment. A full colour version of this figure is available at the British Journal of Cancer journal online.