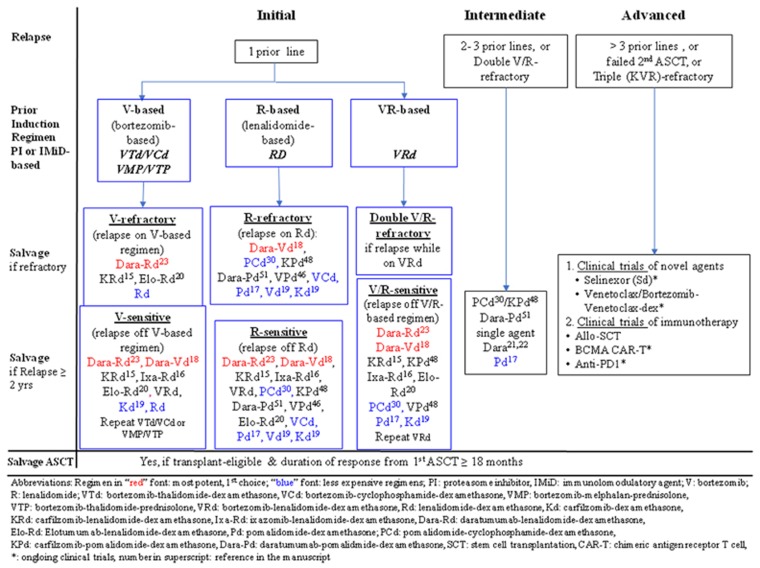
Figure 3.
Proposed algorithm for the treatment of ‘initial’, ‘intermediate’ or ‘advanced’ myeloma relapses. Those with ‘initial’ relapse may have been treated with bortezomib (V)-based, lenalidomide (R)-based, or VR-based induction regimens. In ‘initial’ relapse, in which patients had prior bortezomib-based induction regimens including VTd, VCd, VMP or VTP, if they are bortezomib-refractory, the first choice of salvage is Dara-Rd (red font), followed by KRd. In resource-restricted countries, doublets such as Rd is feasible (blue font). On the other hand, if patients are only bortezomib-exposed but not resistant, Dara-Rd and Dara-Vd (red font) are the most potent options, followed by other PI-based triplets (KRd, Ixa-Rd or VRd), or less expensive doublet (Kd, Rd) (blue font). Moreover, one may repeat the original regimen if the duration of response is ⩾2 years. In those who relapse from Rd induction, if they are R-refractory, pomalidomide combinations (PCd, VPd, Pd), second-generation PI-combinations (KPd, Kd) or V-based regimens (Dara-Vd, VCd, Vd) are viable options. By contrast, in those R-sensitive relapse, Dara-Rd and Dara-Vd are the most potent options, followed by KRd, Ixa-Rd, VRd, Elo-Rd, pomalidomide combinations (PCd, KPd, VPd), or less expensive regimens such as VCd, Vd or Kd (blue font). For patients who relapse on VRd induction, they are double V- and R-refractory, hence managed as ‘intermediate’ relapse. However, if they relapse 2 years after VRd induction, they remain V- and R-sensitive; hence, both V- and R-containing regimens will remain viable. Therefore, Dara-Rd and Dara-Vd are first choices, followed by KRd, Ixa-Rd, Elo-Rd, in addition to pomalidomide-containing regimens such as KPd, VPd, or to repeat VRd. In the ‘intermediate’ relapse category, who may be double V/R-refractory, pomalidomide, daratumumab and carfilzomib-combinations are the main options in addition to single agent daratumumab. In ‘advanced’ relapse, clinical trials of agents with novel mechanism including exportin-1 inhibitor (selinexor combinations), or BCL2 inhibitor (venetoclax combinations) in addition to immunotherapy (anti-BCMA CAR-T cells, allo-HSCT or anti-PD1 antibodies) have to be considered.