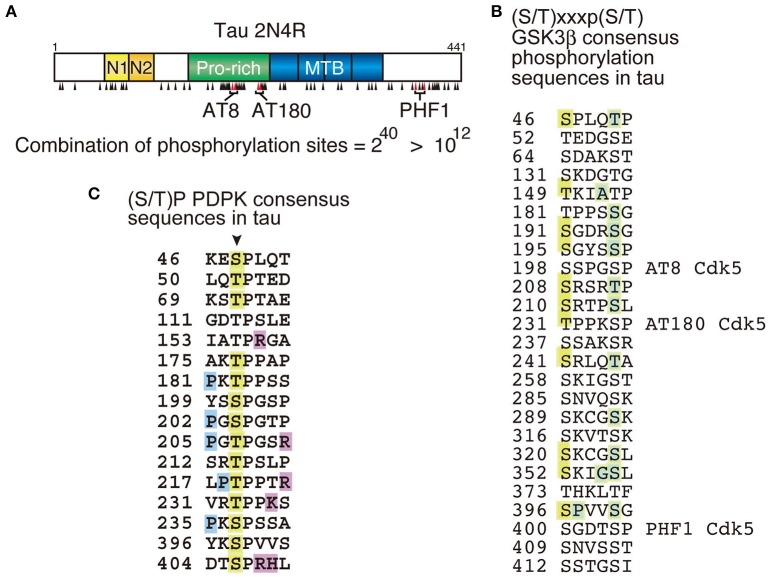
Figure 1.
Phosphorylation sites in tau molecule. (A) The longest human tau isoform is composed of 441 amino acids with four microtubule-binding (MTB) repeats in the C-terminal half. Phosphorylation sites are indicated by black arrowheads. AT8 (Ser202 and Thr205), AT180 (Thr231 and Ser235) and PHF1 (Ser396 and Ser404) are phosphospecific antibodies frequently used for the postmortem diagnosis of tauopathy and their epitopes are indicated. The number of phosphorylation combination, if all sites are phosphorylated independently, is indicated below. (B) Amino acid sequences conforming to the GSK3β consensus sequences, (S/T)xx(x)p(S/T), in tau. There are 25 such sequences and 12 sites are reported to be phosphorylated (orange). The site in the C-terminal sides known to be phosphorylated are indicated by green. (C) Ser/Thr-Pro {(S/T)P} sequences in tau targeted by proline-directed protein kinases (PDPK). Arrow indicates Ser or Thr in (S/T)P sequences. Orange is the reported phosphorylation sites, blue is proline (P) conforming to the consensus sequence {Px(S/T)P or P(S/T)P} for MAPK, and magenta is basic amino acids at the C-terminal site which makes Ser or Thr phosphorylation sites favorable for Cdk5.