Figure 3.
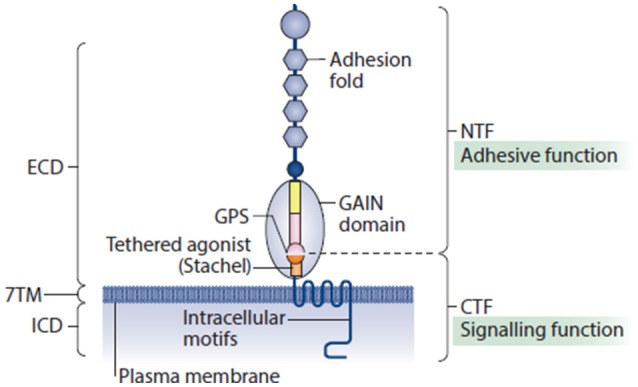
Adhesion G protein-coupled receptors possess structural elements of adhesion molecules and GPCRs. Their extended extracellular domain (ECD) usually contains a collection of adhesion motifs that can engage with cellular and matricellular interaction partners, and a juxtamembrane GPCR autoproteolysis-inducing (GAIN) domain, which is present in all aGPCRs. GAIN subdomain A (yellow rectangle), GAIN subdomain B (pink rectangle) and the GPCR proteolysis site (GPS) motif (pink and orange semicircles) are shown. The GAIN domain is directly connected to the seven-transmembrane (7TM) unit through a linker sequence of approximately 20 amino acids, known as the Stachel (stalk). Recently, this structural component of aGPCRs was identified as a tethered agonist, which stimulates metabotropic activity of several aGPCR homologs. Similar to the ECD, the intracellular domains (ICDs) of aGPCRs can be unusually large. It is estimated that more than one-half of all known aGPCRs undergo auto-proteolytic cleavage that is catalyzed through the GAIN domain, which is present on the cell surface as a non-covalent heterodimer between an amino-terminal fragment (NTF) and a carboxy-terminal fragment (CTF). The cleavage occurs at the evolutionarily highly conserved GPS. Reprinted by permission from Macmillan Publishers Ltd: (Langenhan et al., 2016).
