Abstract
Postnatal antiretroviral (ARV) prophylaxis for infants born to women with HIV is a critical component of perinatal HIV transmission prevention. However, variability in prophylaxis regimens remains and consistency with guidelines has not been evaluated in the United States. We evaluated trends over time in prophylaxis regimens among 6386 HIV-exposed uninfected (HEU) infants using pooled data spanning two decades from three US-based cohorts: the Women and Infants Transmission Study (WITS, 1990–2007), Pediatric AIDS Clinical Trials Group (PACTG) 219C (1993–2007), and the PHACS Surveillance Monitoring of ART Toxicities (SMARTT) study (2007–2015). We also identified maternal and infant risk factors for use of combination prophylaxis regimens (≥2 ARVs) and examined consistency with US perinatal guidelines. We found that receipt of combination prophylaxis between 1996 and 2015 ranged from 2% to 15%, with a consistent median duration of 6 weeks. Infants whose mothers had lower CD4 T-cell counts, higher viral load (VL), no antepartum ARVs, age <20 years at delivery, and Cesarean delivery had significantly higher rates of combination prophylaxis, while infants born 2006–2010 (vs. 2011–2015), who were Hispanic or with lower maternal education levels, had significantly lower rates. Predictors for combination prophylaxis varied over time, with the strongest associations of maternal VL in later birth cohorts. While use of combination prophylaxis increased over time, only 50% of high-risk infants received such regimens in 2011–2015. In conclusion, HEU infants at higher risk of HIV acquisition are more likely to receive combination neonatal prophylaxis, consistent with US guidelines. However, substantial variability remains, and infants at higher risk often fail to receive combination prophylaxis.
Keywords: : neonatal prophylaxis, pregnancy, viral load, antiretrovirals, HIV-exposed uninfected infants
Introduction
Use of combination antiretroviral (ARV) therapy for prevention of mother-to-child HIV transmission and for treatment of HIV-infected pregnant women has contributed to a substantial reduction in the number of HIV-infected infants.1 In addition to prenatal maternal ARVs, US guidelines have recommended that infants of HIV-infected women receive postnatal zidovudine (ZDV) prophylaxis for 6 weeks based on the Pediatric AIDS Clinical Trials Group (PACTG) 076 study findings.2,3 Starting in 2000, infants of mothers receiving ZDV plus nevirapine (NVP) in the peripartum period were recommended to receive ZDV plus a single dose of NVP (ZDV+SD NVP).2 Despite the key role such neonatal prophylaxis plays in HIV prevention, there are few randomized studies on which to base these guidelines; many of the studies previously conducted included treatment arms consisting of antepartum, intrapartum, and postpartum approaches for HIV prevention making it difficult to distinguish the specific effects of postnatal ARV prophylaxis.
More recent US guidelines recommend combination prophylaxis for higher risk infants whose mothers received neither antepartum nor intrapartum ARV drugs, received only intrapartum ARV drugs, or received antepartum drugs but had unsuppressed viral load (VL).2 HIV providers may also consider other criteria, including late HIV diagnosis, poor maternal ARV adherence, or documented maternal resistance to ZDV.4 Based on the only randomized trial to compare combination prophylaxis regimens, ZDV plus three doses of NVP in the first week of life are currently recommended in infants whose mothers received no antepartum ARVs.5 A three-drug regimen of ZDV, lamivudine (3TC), and NVP is also noted as an alternative option for higher risk infants.2
European providers have more often utilized combination prophylaxis in newborns, but have also allowed for shorter durations.6–9 In the United Kingdom and Ireland, Haile-Selassie et al. reported that between 2001–2008, 3% of infants received dual ARV prophylaxis and 11% received triple-drug prophylaxis.9 Canadian studies have also provided additional support for combination regimen use in high-risk infants, indicating few major toxicity concerns.10 German–Austrian recommendations suggest 2–4 weeks of ZDV for low-risk infants rather than a 6-week duration.11 Since 2013, US perinatal guidelines allow for 4-week prophylaxis duration for “low-risk” infants (full-term, born to adherent mothers on combination ARV regimen, with suppressed VL).2 However, a lack of consensus regarding neonatal prophylaxis persists, with variability across Western European cohorts between 1996 and 2010 in rates of receiving no neonatal prophylaxis (0–28.8%), combination prophylaxis (5.3–29.4%), and duration of prophylaxis.6,12
A better understanding of predictors of neonatal prophylaxis regimens may promote intervention or educational efforts to reduce HIV transmission.13,14 In addition, better characterization of predictors of postnatal ARV exposures will allow for more informed assessment of the association of such postnatal regimens with subsequent child health outcomes. The objectives of our study were to characterize the use and duration of postnatal ARV prophylaxis in uninfected infants born to HIV-infected women in the United States, using three well-characterized cohorts conducted over two decades; to identify predictors of combination neonatal prophylaxis and to assess consistency with US recommendations regarding neonatal prophylaxis.
Materials and Methods
Description of protocols and study population
We included HIV-exposed uninfected (HEU) infants born to HIV-infected women from three multisite prospective cohorts conducted at clinical research sites in the United States, including Puerto Rico: the Women and Infants Transmission Study (WITS) conducted from 1990 to 2007, the PACTG 219C study conducted from 1993 to 2007, and the Surveillance Monitoring of ART Toxicities (SMARTT) study conducted by the Pediatric HIV/AIDS Cohort Study (PHACS) network from 2007 to the present. All three studies included objectives of evaluating the safety of ARV medications prescribed for the prevention of mother-to-child HIV transmission. The WITS enrolled HIV-infected women during pregnancy at six sites and included 2470 HEU children.15,16 The PACTG 219C study enrolled 1790 HEU infants at over 60 clinical research sites.17,18 The SMARTT study is conducted at 22 sites and includes a static cohort, which enrolled mothers or caregivers and their children 1–12 years old at entry with information from past studies on in utero ARV exposures, and a dynamic cohort, which enrolls women and their infants prospectively during pregnancy or within 1 week after delivery. The static cohort closed to accrual in 2009 (with 1240 HEU children), while the dynamic cohort continues enrollment (with 2388 HEU infants enrolled as of April 1, 2016).19,20 Both the static and dynamic cohorts were included in our analysis. All three studies were approved by local Institutional Review Boards and written informed consent was obtained from each parent or legal guardian. Some infants participated in more than one study, but overlap was eliminated before analysis so that each unique infant was represented only once. Neither the PACTG 219C study nor the SMARTT study was designed to evaluate perinatal HIV transmission, and thus our analysis included only uninfected infants.
The WITS and SMARTT studies enrolled mother–infant pairs, while 219C enrolled only the children and collected minimal data on maternal ARVs and health measures. For the SMARTT and WITS studies, information on maternal HIV-related measures was collected during pregnancy and/or at study entry. This information included the mothers' ARV regimen(s) during pregnancy, maternal VL, and CD4 count, and HIV diagnosis within 2 months before delivery. Maternal demographic and other health-related information was also collected, including maternal age at delivery, education and income level, substance use (alcohol, tobacco, illicit drugs), and sexually transmitted infections (STIs). Race and ethnicity were self-reported by the mothers and categorized as white non-Hispanic, black non-Hispanic, Hispanic, or “other.” Birth characteristics were abstracted from medical charts and included mode of delivery (Cesarean section or vaginal), gestational age, birth weight, and occurrence of obstetrical complications.
Neonatal ARV prophylaxis
The outcome of interest was the type of neonatal ARV prophylaxis regimen reported for each infant in the first 8 weeks of life. This outcome was dichotomized as combination prophylaxis, defined as two or more ARV drugs, compared with either ZDV+SD NVP or ZDV (or other) monotherapy. Infants receiving more than one type of prophylaxis regimen were classified according to their most intensive regimen. No information on dosages of specific ARV drugs was collected in these studies. Our primary analyses compared combination prophylaxis regimens to monotherapy (ZDV or other ARV alone, or ZDV+SD NVP), but secondary analyses were also conducted, which included ZDV+SD NVP as a combination regimen. All types of neonatal regimens were descriptively summarized, and use of individual ARV drugs included in combination regimens was examined.
Statistical methods
We calculated the percent of infants receiving combination ARV regimens overall and by birth year, including infants from all three studies. For infants enrolled in the SMARTT and PACTG 219C study, we also summarized the percent receiving combination prophylaxis by clinical research site. Due to the limited availability of maternal health measures in the PACTG 219C study, evaluation of predictors of combination neonatal prophylaxis was limited to data from the SMARTT and WITS studies and was restricted to infants receiving prophylaxis born after 1995. We estimated adjusted prevalence ratios (aPRs) and 95% confidence intervals (CIs) for use of combination prophylaxis regimens based on maternal and child risk factors using modified Poisson regression models with robust variances.21 Missing indicators were used for unknown household income and unknown STI status. Models were fit overall and stratified by birth cohort (1996–2000, 2001–2005, 2006–2010, and 2011–2015). To evaluate the association of neonatal combination prophylaxis with maternal ARV regimen, we considered both the type of ARV regimen—categorized as no ARVs or intrapartum only, highly active antiretroviral therapy (HAART, defined as at least three ARV drugs from two drug classes), or non-HAART regimen—and the timing of ARV regimen, categorized as late ARV initiation (third trimester) or no ARV initiation compared to initiation during the first or second trimester.
A number of sensitivity analyses were conducted, including controlling for the correlation among children born to the same mother using a generalized estimating equation model for repeated measures, controlling for within-site correlation (restricted to the SMARTT study only), and excluding the SMARTT static cohort infants to avoid potential recall bias, since information on maternal ARV is reported retrospectively for some static infants.
Results
Characteristics of the study population
The SMARTT, 219C, and WITS studies enrolled a total of 7896 HEU infants. After removing repeat information for 756 infants represented in more than one of these studies, 7140 unique infants remained. Among these, 754 infants were excluded due to lack of detailed maternal or neonatal ARV information; most (733 of 754) had not yet attended their 1-year SMARTT visit, at which neonatal prophylaxis regimens are completed. Our analysis thus included 6386 unique uninfected infants with available neonatal ARV information (WITS: 2464; 219C: 1653; SMARTT: 2269). Demographic characteristics are presented in Table 1; overall, 55% were black non-Hispanic and 33% were Hispanic. Prevalence of low birth weight and preterm birth remained relatively consistent across the three cohorts (15–20%), but delivery by Cesarean section increased from 35% during the WITS study period to 56% in the more recent SMARTT study. Maternal alcohol and illicit drug use were substantially lower in the SMARTT study than for the earlier WITS (10% vs. 24%).
Table 1.
Demographic Characteristics, Maternal Health Measures, and Birth Characteristics of HIV-Exposed Uninfected Infants By Study Cohort
| Study, n (%) | ||||
|---|---|---|---|---|
| Characteristica | Total (N = 6386), n (%) | WITS (N = 2464) | 219C (N = 1653) | SMARTT (N = 2269) |
| Male infant sex | 3233 (51) | 1253 (51) | 819 (50) | 1161 (51) |
| Race/ethnicity | ||||
| White Non-Hispanic | 550 (9) | 269 (11) | 162 (10) | 119 (5) |
| Black Non-Hispanic | 3534 (55) | 1190 (48) | 954 (58) | 1390 (61) |
| Hispanic (regardless of race) | 2106 (33) | 867 (35) | 500 (30) | 739 (33) |
| Other/unknown | 196 (3) | 138 (6) | 37 (2) | 21 (1) |
| Birth year | ||||
| ≤1995 | 961 (15) | 837 (34) | 124 (8) | — |
| 1996–2000 | 1695 (27) | 1019 (41) | 633 (38) | 43 (2) |
| 2001–2005 | 1688 (26) | 608 (25) | 893 (54) | 187 (8) |
| 2006–2010 | 1139 (18) | — | 3 (<1) | 1136 (50) |
| >2010 | 903 (14) | — | — | 903 (40) |
| Preterm birth (<37 weeks gestation) | 1108 (17) | 419 (17) | 242 (15) | 447 (20) |
| Low birth weight (≤2500 g) | 1119 (18) | 425 (17) | 285 (17) | 409 (18) |
| Delivery by cesarean section | 2862 (45) | 856 (35) | 732 (44) | 1274 (56) |
| Maternal age at delivery <20 years | 450 (7) | 174 (7) | 139 (8) | 137 (6) |
| Maternal education< high school graduate | 2249 (35) | 832 (34) | 651 (39) | 766 (34) |
| Household annual income ≤$20K | 3465 (54) | 1839 (75) | N/A | 1626 (72) |
| Maternal substance use during pregnancy | ||||
| Tobacco | 1558 (24) | 913 (37) | 240 (15) | 405 (18) |
| Alcohol | 1154 (18) | 841 (34) | 120 (7) | 193 (9) |
| Illicit drugs | 1270 (20) | 824 (33) | 250 (15) | 196 (9) |
| Maternal health characteristics at delivery | ||||
| HIV viral load >1000 copies/mL | 1504 (24) | 1248 (51) | N/A | 256 (11) |
| CD4 < 350 (cells/mm3) | 1320 (21) | 728 (30) | N/A | 592 (26) |
| Maternal HIV diagnosed within 2 months of delivery | 127 (2) | 105 (4) | N/A | 22 (1) |
| ARV regimen during pregnancy | ||||
| No ARV | 595 (9) | 454 (18) | 96 (6) | 45 (2) |
| Non-HAART | 1802 (28) | 1021 (41) | 571 (35) | 210 (9) |
| HAART | 3774 (59) | 896 (36) | 924 (56) | 1954 (86) |
| Unknown | 215 (3) | 93 (4) | 62 (4) | 60 (3) |
| Time initiating ARV during pregnancy | ||||
| No ARV | 595 (9) | 454 (18) | 96 (6) | 45 (2) |
| 1st trimester | 2417 (38) | 598 (24) | 596 (36) | 1223 (54) |
| 2nd trimester | 2361 (37) | 873 (35) | 665 (40) | 823 (36) |
| 3rd trimester | 788 (12) | 436 (18) | 234 (14) | 118 (5) |
| Time initiating HAART during pregnancy | ||||
| No ARV | 595 (9) | 454 (18) | 96 (6) | 45 (2) |
| Non-HAART | 1802 (28) | 1021 (41) | 571 (35) | 210 (9) |
| 1st trimester | 1764 (28) | 299 (12) | 351 (21) | 1114 (49) |
| 2nd trimester | 1521 (24) | 390 (16) | 407 (25) | 724 (32) |
| 3rd trimester | 489 (8) | 207 (8) | 166 (10) | 116 (5) |
Some characteristics were not available for all participants in addition to those specifically noted as unknown or not available (N/A) above, including: preterm birth (n = 67, 1%), low birth weight (n = 82, 1%), mode of delivery (n = 256, 4%), maternal age at delivery (n = 97, 2%), maternal education (n = 173, 3%), household income (n = 345, 12% WITS, 2% SMARTT), maternal substance use during pregnancy (n = 138–144, 2%), timing of maternal HIV diagnosis (n = 1331, 7% WITS, 52% SMARTT), and timing of ARV initiation during pregnancy (n = 225, 4%). Percentages are reported among all participants in each cohort.
ARV, antiretroviral; HAART, highly active antiretroviral treatment; N/A, not available; SMARTT, Surveillance Monitoring of ART Toxicities; WITS, Women and Infant Transmission Study.
Types of neonatal ARV prophylaxis
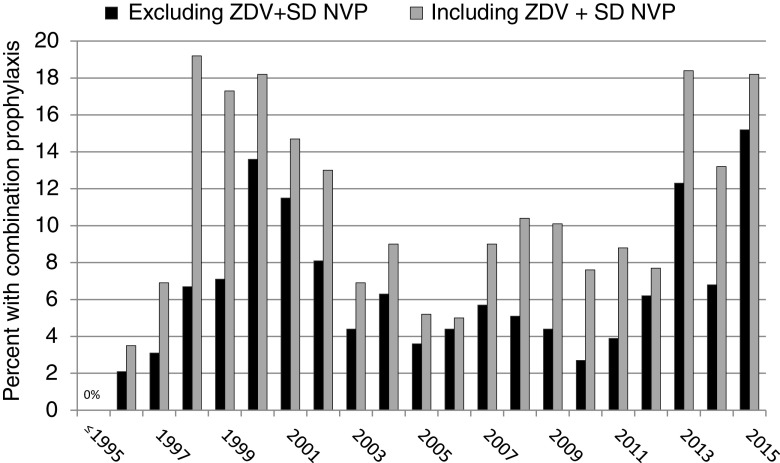
Overall, 568 of the 6386 infants (8.9%) received no neonatal prophylaxis, 5200 (81.4%) received monotherapy (99% of these were ZDV monotherapy), 253 (4.0%) received ZDV+SD NVP, and 365 (5.7%) received a combination postnatal newborn ARV regimen (≥2 drugs excluding ZDV+SD NVP) (Table 2). The 568 infants receiving no prophylaxis were generally born before 1996. The percentage receiving combination prophylaxis was 0% before 1996, increased to as high as 13% in 2000, decreased to ∼4% between 2003 and 2009, and then ranged from 6% to 15% between 2012 and 2015 (Fig. 1). Use of combination neonatal prophylaxis varied substantially across research sites, ranging from 0% to 14% across the 22 SMARTT sites, and from 0% to 100% at the 67 included PACTG 219C sites (data not shown). When those receiving ZDV+SD NVP were combined with infants receiving combination prophylaxis, the prevalence was as high as 24% at SMARTT sites. Among the 365 infants receiving a combination regimen, 133 (36%, or 2.1% of all infants) received at least one 3-drug postnatal regimen, with the most common regimens consisting of ZDV +3TC with NVP, nelfinavir (NFV), or stavudine (d4T). The most common two-drug combination regimens included ZDV +3TC or ZDV+NVP (Table 2).
Table 2.
Types of Neonatal Antiretroviral Prophylaxis Received By 6386 Newborns in the First 8 Weeks of Life
| Type of neonatal ARV prophylaxis | ARV prophylaxis regimen | N (%) |
|---|---|---|
| No ARV prophylaxis | 568 (8.9) | |
| Monotherapy | 5200 (81.4) | |
| Zidovudine alone | ZDV | 5170 (81.0) |
| Other monotherapy (n = 30) | Stavudine (d4T) | 18 (0.3) |
| Nevirapine (NVP) | 9 (0.1) | |
| Lamivudine (3TC) | 2 (<0.1) | |
| Ritonavir (RTV) | 1 (<0.1) | |
| ZDV+single-dose NVP | ZDV+SD NVP | 253 (4.0) |
| Combination ARV prophylaxisa | 365 (5.7) | |
| Ever on 2-drug regimen | 317 (5.0) | |
| Ever on 3-drug or 4-drug regimen | 133 (2.1) |
| Specific regimens for combination prophylaxis | N (% among category) | |
|---|---|---|
| 2-drug regimens | ZDV +3TC | 219 (66.8) |
| (328 regimens received by 317 unique newborns) | ZDV+NVP | 85 (25.9) |
| 3TC+nelfinavir (NFV) | 4 (1.2) | |
| ZDV+abacavir (ABC) | 3 (0.9) | |
| ZDV+didanosine (ddI) | 3 (0.9) | |
| ZDV+d4T | 2 (0.6) | |
| ZDV+NFV | 2 (0.6) | |
| 3TC+d4T | 2 (0.6) | |
| Other 2-drug regimens | 8 (2.4) | |
| 3-drug or 4-drug regimens | ZDV+3TC+NVP | 94 (65.3) |
| (144 regimens received by 133 unique newborns) | ZDV+3TC+NFV | 18 (12.5) |
| ZDV+3TC+d4T | 7 (4.8) | |
| ZDV+3TC+lopinavir/ritonavir (LPV/r) | 4 (2.8) | |
| ZDV+emtricitabine (FTC)+tenofovir disoproxil fumarate (TDF) | 4 (2.8) | |
| 3TC+d4T+NVP | 2 (1.4) | |
| Other 3-drug regimens | 5 (3.5) | |
| ZDV+3TC+NVP+NFV | 3 (2.1) | |
| ZDV+3TC+NVP+LPV/r | 2 (1.4) | |
| ZDV+3TC+NVP+raltegravir (RAL) | 2 (1.4) | |
| Other 4-drug regimens | 3 (2.1) |
Note that infants may have received multiple neonatal prophylaxis regimens during the first 8 weeks and thus percentages may not add to 100%.
3TC, lamivudine; d4T, stavudine; LPV/r, lopinavir/ritonavir; NFV, nelfinavir; SD NVP, single-dose nevirapine; ZDV, zidovudine.
FIG. 1.
Percent of infants receiving combination neonatal ARV prophylaxis (at least 2 ARV medications) by calendar year. ARV, antiretroviral.
The percent of WITS and SMARTT infants classified as “higher risk” based on maternal VL >1000 copies/mL and/or lack of maternal antepartum (or only intrapartum) ARVs decreased over time from 48% in 1996–2000 to 9.5% in 2011–2015 (Table 3). At the same time, the percent of high-risk infants receiving combination prophylaxis increased from 5.3% in 1996–2000 to 29.1% in 2011–2015; consideration of ZDV+SD NVP as a combination regimen further increased these percentages of high-risk infants receiving combination prophylaxis to 50% in 2011–2015 (Table 3).
Table 3.
Receipt of Combination Prophylaxis Among SMARTT and WITS Infants Identified as High-Risk Based on Maternal Viral Load and Antepartum Antiretroviral Regimen
| Birth year | Total number of infants born | High-risk infants,a N (%) | Combination prophylaxisb(excluding ZDV+SD NVP) in high-risk infants, N (%) | Combination prophylaxisb(including ZDV+SD NVP) in high-risk infants, N (%) |
|---|---|---|---|---|
| 1996–2000 | 1062 | 507 (47.7) | 27 (5.3) | 36 (7.1) |
| 2001–2005 | 795 | 151 (19.0) | 18 (11.9) | 27 (17.9) |
| 2006–2010 | 1136 | 165 (14.5) | 21 (12.7) | 37 (22.4) |
| >2010 | 903 | 86 (9.5) | 25 (29.1) | 43 (50.0) |
High-risk defined here as maternal viral load >1000 copies/mL before labor or delivery, and/or lack of antepartum ARV therapy (or intrapartum only).
Combination prophylaxis defined as two or more ARV drugs used together in the first 8 weeks of life.
ARV, antiretroviral; SD NVP, single-dose nevirapine; SMARTT, Surveillance Monitoring of ART Toxicities; WITS, Women and Infant Transmission Study; ZDV, zidovudine.
Duration of neonatal ARV prophylaxis
The median duration of prophylaxis was 6 weeks across all four birth cohorts, and 65% of infants received between 5 and 7 weeks overall, while 85% received 5–7 weeks since 2006. In 2014, guidelines began to recommend a shorter course of ZDV alone for low-risk infants.2 Among the 238 infants born in 2014 or later, 154 (65%) were classified as “low risk” and 21 (13.6%) received a 4-week ZDV prophylaxis regimen. This was only slightly higher than the 10.1% who received a 4-week ZDV monotherapy regimen among the 69 infants not classified as low risk. Some infants receiving shorter courses had contraindications for abbreviated regimens, including preterm birth (n = 5), lack of maternal combination ARV therapy during pregnancy (n = 2), and maternal VL >400 copies/mL before labor and delivery (n = 1).
Predictors of combination neonatal ARV prophylaxis
A summary of predictors of receipt of combination neonatal prophylaxis in the SMARTT and WITS cohorts is presented in Table 4, including both unadjusted and aPRs. Infants born between 2006 and 2010 were significantly less likely to receive combination prophylaxis than those born after 2010. In multivariable adjusted models, both low maternal CD4 count and unsuppressed VL were associated with increased prevalence, although maternal CD4 count showed stronger (and significant) associations. Infants delivered via Cesarean section also had significantly higher prevalence of combination prophylaxis. Infants of mothers without antepartum ARVs or receiving only intrapartum ARVs had over twofold higher prevalence of combination prophylaxis, even after adjusting for maternal immunologic and virologic status at delivery. Infants whose mothers were younger than 20 years of age at delivery were more likely to receive combination prophylaxis, while Hispanic infants had significantly reduced prevalence.
Table 4.
Predictors of the Prevalence of Receiving Combination Antiretroviral Prophylaxis as Newborn Regimen
| Univariablea | Multivariable | |||
|---|---|---|---|---|
| Predictor | Prevalence ratio (95% CI) | p | Prevalence ratio (95% CI) | p |
| Birth year (ref: >2010) | ||||
| 1996–2000 | 1.04 (0.77–1.41) | 0.80 | 0.95 (0.61–1.47) | 0.81 |
| 2001–2005 | 1.02 (0.73–1.41) | 0.93 | 1.03 (0.72–1.49) | 0.86 |
| 2006–2010 | 0.59 (0.41–0.84) | 0.003 | 0.56 (0.39–0.81) | 0.002 |
| Maternal viral loadb (copies/mL) (ref: ≤400 copies/mL) | ||||
| >1000 | 1.86 (1.44–2.39) | <0.001 | 1.40 (1.00–1.96) | 0.05 |
| 401–1000 | 1.65 (1.07–2.53) | 0.02 | 1.51 (0.95–2.41) | 0.08 |
| Maternal CD4 countb (cells/μL) (ref: ≥350 cells/μL) | ||||
| <200 | 2.23 (1.62–3.06) | <0.001 | 2.02 (1.45–2.83) | <0.001 |
| 200–349 | 1.73 (1.31–2.30) | <0.001 | 1.70 (1.27–2.28) | <0.001 |
| Time initiating ARV regimen (ref: 1st or 2nd trimester) | ||||
| No ARV | 2.46 (1.41–4.30) | 0.002 | 2.24 (1.13–4.46) | 0.02 |
| Late ARV (3rd trimester) | 1.13 (0.78–1.62) | 0.52 | 0.86 (0.56–1.32) | 0.49 |
| Cesarean delivery (vs vaginal) | 1.54 (1.21–1.97) | <0.001 | 1.54 (1.18–2.01) | 0.002 |
| Age of motherb (ref: 20–34 years) | ||||
| ≥35 years | 0.97 (0.70–1.35) | 0.87 | 0.92 (0.65–1.31) | 0.65 |
| <20 years | 1.37 (0.91–2.06) | 0.14 | 1.82 (1.19–2.80) | 0.01 |
| Low maternal education (<high school graduate) | 0.77 (0.59–1.00) | 0.05 | 0.78 (0.58–1.03) | 0.08 |
| Race/ethnicity (ref: black non-Hispanic) | ||||
| Hispanic | 0.50 (0.37–0.68) | <0.001 | 0.54 (0.39–0.75) | <0.001 |
| White/other non-Hispanic | 1.37 (0.97–1.92) | 0.07 | 1.36 (0.92–2.01) | 0.13 |
Combination prophylaxis defined as at least two ARV drugs received in the first 8 weeks of life, compared to either monotherapy (primarily ZDV) or ZDV+SD NVP.
Univariable associations are only shown for those predictors retained in the final adjusted multivariable model.
Latest measure before or at labor/delivery.
ARV, antiretroviral; CI, confidence interval; SD NVP, single-dose nevirapine; ZDV, zidovudine.
Sensitivity analyses accounting for correlation among multiple children born to the same mother yielded almost identical results. Exclusion of SMARTT Static participants also yielded similar findings. Sensitivity analyses accounting for correlation among infants at the same research site were restricted to the SMARTT study (N = 2259), and as a result tended to include a higher percentage of infants born in the most recent periods; unsuppressed maternal VL showed even more striking associations with combination neonatal prophylaxis in this subgroup (aPR = 4.09, 95% CI 2.57–6.51 for VL >1000 copies/mL and aPR = 2.91, 95% CI 1.45–5.87 for 400–1000 copies/mL compared with VL <400 copies/mL).
In secondary analyses including ZDV+SD NVP with combination prophylaxis, findings were generally similar (Table 5); however, unsuppressed VL showed stronger associations with receipt of combination prophylaxis than did low CD4 counts. Lack of maternal receipt of ARVs and maternal age at delivery were no longer identified as significant predictors of combination prophylaxis after adjustment for maternal health status at delivery and other covariates.
Table 5.
Adjusted Model for Prevalence of Receiving Combination Antiretroviral Prophylaxis as Newborn Regimen: Sensitivity Analysis Including ZDV+SD NVP as a Combination Regimen
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Covariate | Prevalence ratioa(95% CI) | p | Prevalence ratio (95% CI) | p |
| Birth year (ref: >2010) | ||||
| 1996–2000 | 0.81 (0.63–1.04) | 0.10 | 0.63 (0.44–0.89) | 0.01 |
| 2001–2005 | 0.81 (0.61–1.06) | 0.12 | 0.73 (0.54–0.98) | 0.04 |
| 2006–2010 | 0.72 (0.56–0.93) | 0.01 | 0.66 (0.51–0.86) | 0.002 |
| Maternal viral loadb (copies/mL) (ref: ≤400 copies/mL) | ||||
| >1000 | 2.02 (1.65–2.47) | <0.001 | 1.96 (1.51–2.54) | <0.001 |
| 401–1000 | 1.61 (1.13–2.29) | 0.01 | 1.58 (1.07–2.33) | 0.02 |
| Maternal CD4 countb (cells/μL) (ref: ≥350 cells/μL) | ||||
| <200 | 1.98 (1.52–2.58) | <0.001 | 1.60 (1.21–2.11) | 0.001 |
| 200–349 | 1.74 (1.39–2.18) | <0.001 | 1.62 (1.29–2.04) | <0.001 |
| Time initiating ARV regimen (ref: 1st or 2nd trimester) | ||||
| No ARV | 2.06 (1.28–3.32) | 0.003 | 1.52 (0.81–2.82) | 0.19 |
| Late ARV (3rd trimester) | 1.05 (0.78–1.42) | 0.74 | 0.94 (0.67–1.32) | 0.73 |
| Cesarean delivery (vs vaginal) | 1.53 (1.25–1.86) | <0.001 | 1.45 (1.17–1.81) | <0.001 |
| Maternal gonorrhea during pregnancy | 1.49 (0.91–2.44) | 0.11 | 1.51 (0.95–2.38) | 0.08 |
| Race/ethnicity (ref: back non-Hispanic) | ||||
| Hispanic | 0.59 (0.47–0.74) | <0.001 | 0.64 (0.50–0.82) | <0.001 |
| White non-Hispanic | 1.16 (0.87–1.56) | 0.32 | 1.20 (0.85–1.68) | 0.30 |
Combination prophylaxis defined as at least two ARV drugs received in the first 8 weeks of life, or ZDV+SD NVP, compared to monotherapy (primarily ZDV).
Unadjusted associations are only shown for those predictors retained in the final adjusted multivariable model.
Latest measure before or at labor/delivery
ARV, antiretroviral; CI, confidence interval; ZDV, zidovudine; ZDV+SD NVP, Zidovudine plus single-dose nevirapine.
In evaluating predictors of both the primary and alternate definition of combination prophylaxis, there were no associations observed with preterm birth or low birth weight, with maternal substance use (alcohol, tobacco, or illicit drugs) during pregnancy, or with occurrence of STIs. Obstetrical complications such as preeclampsia, gestational and pregestational diabetes, or opportunistic infections were not collected for over 40% of mothers, but appeared to show little association with combination prophylaxis.
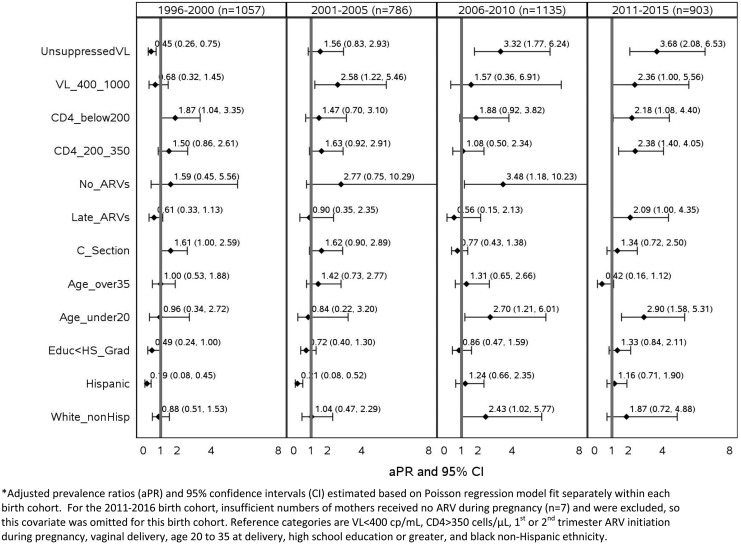
Evaluation of predictors of receipt of neonatal combination prophylaxis by birth cohort demonstrated shifts in predictors, which would be expected given changes in guidelines over time (Fig. 2). Few of the maternal health or ARV-related characteristics other than lower CD4 count were associated with combination prophylaxis before 2001, but unsuppressed VL emerged as a strong predictor among infants born 2001–2005. Both unsuppressed VL (401–1000 and/or >1000 copies/mL) and low CD4 counts showed associations with increased prevalence of combination prophylaxis in the latest two time periods, 2006–2010 and 2011–2015. Delivery by Cesarean section showed stronger associations with combination prophylaxis before 2005, while young maternal age at delivery demonstrated associations only since 2006. Compared with black non-Hispanic infants, Hispanic infants were less likely to receive combination prophylaxis before 2005, while white non-Hispanic infants were more likely since 2006. Infants born to mothers with low education were less likely to receive combination prophylaxis in earlier birth cohorts, but this association was less apparent in the most recent time periods.
FIG. 2.
Adjusted prevalence ratios for receipt of combination neonatal ARV prophylaxis by birth cohort. ARV, antiretroviral.
Discussion
We observed less than 6% of HEU infants receiving combination prophylaxis in the United States over the last 25 years. Although the percentage has increased substantially recently and may suggest increasing uptake of recommendations, half of high-risk infants still fail to receive combination prophylaxis. Our findings were similar to those reported for the IMPAACT P1025 study, where 91% of infants received ZDV alone as neonatal prophylaxis, 3% also received SD NVP, and 6% received other combination ARV prophylaxis.22 However, infants in the United States are much less likely to receive combination prophylaxis than their European counterparts, where up to 14% receive combination prophylaxis.5,8 In addition, only about 2% of infants in our evaluation received a three-drug combination prophylaxis regimen, while use of triple-ARV prophylaxis is relatively common in Europe and Canada.5–11
Consistency with guidelines is somewhat complex to evaluate, given the lack of clear directives; many of the panel recommendations in earlier periods only note that clinicians may consider use of combination regimens and in particular, three-drug regimens, for high-risk infants. Given the lack of randomized trials and other data on which this practice is based, the recommendations encourage case-by-case decisions in consultation with a pediatric HIV specialist. We observed an increase in the percent of high-risk infants receiving combination prophylaxis over time, but even in the most recent birth cohort (since 2011), half of the infants who met at least one criteria for being at high risk of HIV acquisition received only ZDV monotherapy. We also saw an increase in use of combination prophylaxis between 1998 and 2002, particularly when including SD NVP. This increase may have been attributable to the first mention of combination prophylaxis options in the February 2000 perinatal guidelines, along with release of findings from the PETRA and HIVNET 012 trials.2,23–25
While the panel guidelines have specifically allowed a shorter 4-week neonatal ZDV regimen for low-risk infants since 2014, less than 15% of low-risk infants born in this period received the shorter course. At the same time, some infants who did not meet the low-risk criteria were given a 4-week prophylaxis regimen, despite being born preterm or to a mother with unsuppressed VL. The percentage receiving combination prophylaxis in the United States appears to be driven at least, in part, by site practices, given that some sites never provide combination prophylaxis, while others routinely do, particularly when ZDV+SD NVP is considered a combination regimen.
Our evaluation of predictors of combination prophylaxis revealed some expected findings, such as higher prevalence of combination prophylaxis in infants born to mothers with unsuppressed VL or without antepartum or intrapartum ARVs, which show some consistency with US panel recommendations.2 However, we also identified a strong role of low CD4 count at delivery, even after adjustment for maternal VL, antepartum ARVs, and other covariates. Surprisingly, low CD4 count appeared to play as strong a role in predicting combination prophylaxis as maternal VL. We also observed a significantly higher prevalence of receiving combination prophylaxis among infants born to mothers younger than 20 years of age or via Cesarean delivery, often performed in higher risk deliveries. There was also some evidence of racial and ethnic disparities in receipt of combination prophylaxis, with Hispanic infants less likely and white non-Hispanic infants more likely than black non-Hispanic infants to receive such a regimen, even after adjustment for maternal health status and other factors. These disparities may be partly related to healthcare utilization and access to care, and were mostly observed before 2005. Examination of predictors separately within each birth cohort revealed some other time trends in associations; delivery by Cesarean section was only associated with increased prevalence in the earlier time periods and may have served a role as a surrogate for other risk factors such as higher maternal VL, lack of maternal ARV adherence, or late presentation/diagnosis with HIV.
As an analysis of observational data from three separate cohorts, we recognize certain limitations in our evaluation. First, we had little data on maternal health in the PACTG 219C cohort and thus had to exclude it from our evaluation of predictors. Secondly, we lacked information on maternal ARV adherence and drug resistance, which clinicians may have utilized in determining appropriate prophylaxis regimens for the newborn. We included only HEU infants since neither the PACTG 219C nor SMARTT studies evaluated perinatal transmission, yet, perinatally HIV-infected infants likely less often received combination prophylaxis. Our studies do not obtain information on dosages of specific ARV drugs received by infants, which prevents the distinction between three-drug preemptive treatment, which may be used in the hope of achieving a functional cure, versus combination prophylaxis with the lower dosage of NVP typically used in this setting.26 Despite these limitations, the long-time span and large sample size, along with collection of multiple sociodemographic and maternal health measures, allowed a comprehensive evaluation of factors related to use of combination prophylaxis. We focused specifically on the use of neonatal ARV prophylaxis regimens rather than their safety, although prior examinations have generally been reassuring.10,27,28 However, most evaluations were conducted when little variability in neonatal prophylaxis regimens existed, limiting their power and generalizability. As the use of combination regimens expands among higher risk infants, continued monitoring of the safety of these prevention strategies is warranted.
In summary, we observed overall low rates of combination prophylaxis, including those at higher risk, demonstrating inconsistency with current US guidelines. The vast majority of infants in the United States still receive 6 weeks of ZDV monotherapy (with or without SD NVP); this could represent missed HIV prevention opportunities for a small percentage of higher risk infants. Such missed opportunities should be considered within the broader context of other barriers to preventing HIV transmission, including lack of early and repeat HIV testing in pregnancy, inadequate ARV treatment during pregnancy, and breastfeeding.13,14,29,30 However, the reluctance of clinicians to prescribe combination prophylaxis regimens, and particularly three-drug regimens, to higher risk infants may warrant increased education and outreach, given the safety profile of these regimens reported in the literature.6,10 At the same time, appropriate utilization of a shorter 4-week ZDV regimen for lower risk infants should become more widespread.
Contributor Information
Collaborators: for the Pediatric HIV/AIDS Cohort Study
Acknowledgments
We thank the institutions and site staff involved in the conduct of these studies, for the PHACS SMARTT study (see www.phacsstudy.org/About-Us/SMARTT.Acknowledgement), the PACTG 219C study (see www.phacsstudy.org/About-Us/219_219C.Acknowledgments), and WITS, as well as the children and their families who participated in all these studies. Funding: The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) with cofunding from the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Deafness and Other Communication Disorders (NIDCD), Office of AIDS Research (OAR), the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). Data management services were provided by Frontier Science and Technology Research Foundation, and regulatory services and logistical support were provided by Westat, Inc. The PACTG 219/219C study was supported by the Statistical and Data Analysis Center at Harvard T.H. Chan School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement No. 5U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and No. 1U01 AI068616 with the IMPAACT Group.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Little KM, Taylor AW, Borkowf CB, et al. Perinatal antiretroviral exposure and prevented mother-to-child HIV infections in the era of antiretroviral prophylaxis in the United States, 1994–2010. Pediatr Infect Dis J 2017;36:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf (Last accessed June27, 2017)
- 3.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 9194;331:1173–1180 [DOI] [PubMed] [Google Scholar]
- 4.McKeegan K, Rutstein R, Lowenthal E. Postnatal infant HIV prophylaxis: A survey of US practice. AIDS Patient Care STDs 2011;25:1–4 [DOI] [PubMed] [Google Scholar]
- 5.Nielsen-Saines K, Watts DH, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med 2012;366:2368–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiappini E, Galli L, Giaquinto C, et al. Use of combination neonatal prophylaxis for prevention of mother-to-child transmission of HIV infection in European high-risk infants. AIDS 2013;27:991–1000 [DOI] [PubMed] [Google Scholar]
- 7.De Ruiter A, Mercey D, Anderson J, et al. British HIV Association and Children's HIV Association guidelines for the management of HIV infection in pregnant women 2008. HIV Med 2008;9:452–502 [DOI] [PubMed] [Google Scholar]
- 8.Ferguson W, Goode M, Walsh A, et al. Evaluation of 4 weeks' neonatal antiretroviral prophylaxis as a component of a prevention of mother-to-child transmission program in a resource-rich setting. Pediatr Infect Dis J 2011;30:408–412 [DOI] [PubMed] [Google Scholar]
- 9.Haile-Selassie HT, Townsend CL, Tookey PA. Use of neonatal post-exposure prophylaxis for prevention of mother-to-child HIV transmission in the UK and Ireland, 2001–2008. HIV Med 2011;12:422–427 [DOI] [PubMed] [Google Scholar]
- 10.Kakkar FW, Samson L. Vaudry W, et al. Safety of combination antiretroviral prophylaxis in high-risk HIV-exposed newborns: A retrospective review of the Canadian experience. J Int AIDS Soc 2016;19:20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neubert J, Pfeffer M, Borkhardt A, et al. Risk adapted transmission prophylaxis to prevent vertical HIV_1 transmission: Effectiveness and safety of an abbreviated regimen of postnatal oral Zidovudine. BMC Pregnancy Childbirth 2013;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England K, Thorne C. Use of neonatal antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV is decreasing in Western Europe. Clin Infect Dis 2009;48:1797–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitmore SK, Patel-Larson A, Espinoza L, et al. Missed opportunities to prevent perinatal human immunodeficiency virus transmission in 15 jurisdictions in the United States during 2005–2008. Womens Health 2010;50:414.25. [DOI] [PubMed] [Google Scholar]
- 14.Whitmore SK, Taylor AW, Espinoza L, Shouse RL, Lampe MA, Nesheim S. Correlates of mother-to-child transmission of HIV in the United States and Puerto Rico. Pediatrics 2012;129:e74–e81 [DOI] [PubMed] [Google Scholar]
- 15.Sheon AR, Fox HE, Rich KC, et al. The Women and Infants Transmission Study (WITS) of maternal-infant HIV transmission: Study design, methods, and baseline data. J Women's Health 1996;5:69–78 [Google Scholar]
- 16.Pacheco SE, McIntosh K, Lu M, et al. Women and Infants Transmission Study. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: An analysis of the women and infants transmission study. J Infect Dis 2006;194:1089–1097 [DOI] [PubMed] [Google Scholar]
- 17.Brogly S, Abzug M, Watts DH, et al. Birth defects among children born to HIV-infected pregnant women: Pediatric AIDS Clinical Trials protocols 219 and 219C. Pediatr Infect Dis J 2010;29:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams PL, Marino M, Malee K, et al. for the PACTG 219C Team. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics 2010;125:e250–e260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams PL, Seage GR 3rd, Van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol 2012;175:950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griner R, Williams PL, Read JS, et al. ; for the Pediatric HIV/AIDS Cohort Study. In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care STDs 2011;25:385–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G. A modified poisson regression approach for prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 22.Read JS, Huo Y, Patel K, et al. Laboratory abnormalities among HIV-exposed uninfected infants: IMPAACT Protocol P1025. J Pediatric Infect Dis Soc 2012;1:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Archived version from February 25, 2000. Available at: https://aidsinfo.nih.gov/guidelines/archive/perinatal-guidelines (Last accessed December15, 2017)
- 24.Saba J on behalf of the PETRA Trial Study Team. Interim analysis of early efficacy of three short ZDV/3TC combination regimens to prevent mother-to-child transmission of HIV-1: The PETRA trial. In: Sixth Conference on Retroviruses and Opportunistic Infections, Chicago, IL, January1999 (Abstract S-7) [Google Scholar]
- 25.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354:795–802 [DOI] [PubMed] [Google Scholar]
- 26.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia following treatment cessation in an infant. N Engl J Med 2013;369:1828–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giaquinto C, Rampon O, DeRossi A. Antiretroviral therapy for prevention of mother-to-child HIV transmission: Focus on single-dose nevirapine. Clin Drug Investig 2006;26:611–627 [DOI] [PubMed] [Google Scholar]
- 28.Sirois P, Huo Y, Williams PL, et al. for the Pediatric HIV/AIDS Cohort Study. Safety of perinatal exposure to antiretroviral medications: Developmental outcomes in infants. Pediatr Infect Dis J 2013;32:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao C, Golden WC, Anderson JR, Coleman JS. Missed opportunities for repeat HIV testing in pregnancy: Implications for elimination of mother-to-child transmission in the United States. AIDS Patient Care STDs 2017;31:20–29 [DOI] [PubMed] [Google Scholar]
- 30.Peters V, Liu KL, Dominguez K, et al. Missed opportunities for perinatal HIV prevention among HIV-exposed infants born 1996–2000, pediatric spectrum of HIV disease cohort. Pediatrics 2003;111:1186–1191 [PubMed] [Google Scholar]