Abstract
Objectives
The incidence and severity of Clostridium difficile infections has increased rapidly over the past two decades particularly in elderly patients with multiple comorbidities. This study sought to characterize the incidence and risks of these infections in cardiac surgery patients.
Methods
A total of 5,158 patients at 10 Cardiothoracic Surgical Trials Network sites in the US and Canada participated in a prospective study of major infections after cardiac surgery. Patients were followed for infection, readmission, reoperation, or death up to 65 days after surgery. We compared clinical and demographic characteristics, surgical data, management practices, and outcomes for patients with and without C. difficile infections.
Results
C. difficile was the third most common infection observed (0.97%) and was more common in patients with preoperative co-morbidities and complex operations. Antibiotic prophylaxis > 2 days, intensive care unit stay > 2 days, and postoperative hyperglycemia were associated with increased risk of C. difficile infection. Median time to onset was 17 days; 48% of infections occurred post-discharge. Additional length of stay due to infection was 12 days. The readmission and mortality rates, respectively, were three and five times higher in patients with C. difficile infection compared to uninfected patients.
Conclusions
In this large multi-center prospective study of major infections following cardiac surgery, C. difficile infection was encountered in nearly 1% of patients, was frequently diagnosed post-discharge, extended length of stay, and substantially increased mortality. Patients with comorbidities, longer surgery time, extended antibiotic exposure and/or hyperglycemic episodes have an increased risk of C. difficile infection.
Clostridium difficile is a gram-positive, spore-forming anaerobic bacterium that is the most common source of hospital-acquired gastrointestinal infection.1–7 C. difficile infection (CDI) can cause a wide spectrum of illness ranging from mild diarrhea to pseudo-obstruction, pseudomembranous colitis, prolonged ileus, toxic megacolon, large bowel perforation, hemodynamic collapse, multisystem organ failure, shock, and death.3,6,8–10
Both the incidence and severity of CDI have been increasing worldwide since the mid- to late-1990s as evidenced by a variety of single-site, multi-center, and population-based studies.1–5,8,10–27 Though some increase in observed incidence may be due to the adoption of newer, more sensitive nucleic acid amplification tests (NAATs), the rates of colectomy and mortality continue to climb.5–7,10–14,16,18,20,23 Estimates of hospital cost increases directly due to CDI are as high as $77,0001,4,19,20,28 with cumulative annual costs estimated to be greater than $200 million for cardiac surgery patients alone24 and nearly $5 billion for CDIs in acute care facilities in the United States.25,29,30 The United States Centers for Disease Control and Prevention has identified CDI as an important cause of infectious disease death and made its prevention a national priority.25
Advanced age, blood product transfusions, heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, liver disease, diabetes, history of stroke, renal failure, mechanical ventilation, urinary catheters, decubitus ulcers, health care exposure (e.g. hospitalization), and antibiotic exposure have been identified as risk factors for CDI.8,9,24,31–34 Cardiac surgery patients, therefore, would appear to be a particularly vulnerable population, yet the literature has focused primarily on surgical site infections and bloodstream infections in this population.35,36
The Cardiothoracic Surgical Trials Network (CTSN), funded by the National Institutes of Health and the Canadian Institutes of Health Research, recently conducted a large multicenter prospective cohort study with the primary objective of identifying management practices associated with infections occurring within 65 days after cardiac surgery.37 Indeed, this study showed that CDI was the third most common major infection following pneumonia and bloodstream infection. In order to provide a more thorough understanding of CDI and how it affects patients undergoing cardiac surgery, we analyzed this cohort to determine the incidence of CDI and its association with adverse outcomes as well as demographic variables and management practices associated with increased risk of developing CDI.
METHODS
Population
The study cohort included all 5,158 patients (Figure 1, CONSORT Diagram) at 10 Cardiothoracic Surgical Trials Network (CTSN) sites in the United States and Canada who participated in the prospective Management Practices and the Risk of Infection Following Cardiac Surgery study (NCT01089712).37 All patients at the participating sites who had a clinical indication for cardiac surgery, did not have an active systemic infection, were at least 18 years of age, and provided written informed consent were enrolled between February and September 2010. The study was approved by Institutional Review Boards at each participating clinical center and at the data coordinating center (DCC).
Figure 1.
CONSORT Diagram
Data
Demographic data, baseline laboratory values, comorbidities, surgical data, and management practices (e.g. antimicrobial prophylaxis, glycemic control) were collected for the prospective cohort study. Comorbidities collected included hypertension, hypercholesterolemia, diabetes, chronic lung disease, renal insufficiency (history of renal insufficiency/failure and/or serum creatinine > 2.0 preoperatively), congestive heart failure (CHF), history of infective endocarditis, cerebrovascular accident, peripheral vascular disease, congenital heart disease, and valvular heart disease. Surgical characteristics captured included procedure status (elective, urgent, emergent), procedure, type of incision, operative time, use and duration of bypass and circulatory arrest, and blood transfusions.
Participants were followed for up to 65 days to determine incidence of major and minor infections, all-cause mortality, reoperation, and hospital readmission. The 10 major infections included were deep incisional surgical site infection occurring at the primary chest incision; deep incisional surgical site infection (SSI) occurring at a secondary incision site (e.g., saphenous harvest and groin cannulation sites); mediastinitis; infectious myocarditis or pericarditis; endocarditis; cardiac device infection; pneumonia; empyema; Clostridium difficile colitis; and bloodstream infection. CDI was diagnosed according to standard clinical practice at each site. Minor infections were defined as primary and secondary superficial incisional surgical site infections; symptomatic urinary tract infections; and asymptomatic bacteriuria. Infections were classified based on definitions from the Centers for Disease Control and Prevention (CDC) and the National Healthcare Safety Network surveillance38, and all major infections were adjudicated by an Event Adjudication Committee (EAC) that included three infectious disease experts. Infections other than C. difficile in this cohort have been previously described in detail.37,39,40
All data were entered into an electronic data capture system and submitted to the DCC which was responsible for electronic and local monitoring of the data for quality assurance.
Statistical Analysis
Univariable proportional hazards regression models were used to assess differences in patient demographics, operative characteristics, and post-operative management by whether or not a patient ever had a CDI. We adjusted for patient-level risk factors and management factors but not site in order to avoid obscuring risks related to management practices that may vary by site. Patients missing 60 day visits were censored at time of last contact.
Variables with a p-value ≤ 0.20 were then considered when building the multivariable proportional hazards model of time to onset of CDI using a backwards stepwise process. This model included death as a competing risk using the method of Fine and Gray41. Assumptions of the Fine-Gray model were checked by testing for an interaction between time and each of the covariates and by plotting the Schoenfeld-type residuals over time.42
An interaction between diabetes and hyperglycemia was explored as some studies have found a differential risk of hyperglycemia.43–46 Residuals were plotted to confirm continuous numerical variables included in the final model could be treated as linear. AIC values were considered in final model selection.
The cumulative incidence function of CDI was plotted, again treating death as a competing risk.41 A multivariable extended Cox model was also used to assess the relationship between time to death and a time-varying indicator for CDI, adjusting for factors found to be predictive of mortality in the cohort as a whole37 and for other non-C. difficile infection as a time-varying covariate. Survival estimates for the hypothetical average patient in the cohort (age 64 years, creatinine 1.165 mg/dL, no diabetes or heart failure, no other infection) were generated from the proportional hazards regression model and plotted by time-varying CDI status.
A multi-state time-inhomogeneous Markov model47 was used to determine the excess length of stay (LOS) of the index hospitalization due to CDI. The model assumed a single initial state (index surgical procedure), one intermediate state (CDI), and two absorbing states (hospital discharge and death). A bootstrap standard error and 95% confidence interval (CI) for the excess LOS was computed based on 1000 bootstrap samples.
All variables included in the final models had a p-value ≤ 0.05, and all analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R 3.1.1.
RESULTS
Patient Characteristics
The study cohort included a total of 5,158 patients who enrolled in the study and underwent cardiac surgery between February and September 2010. Patient characteristics of the overall cohort have been previously reported37 and are shown in Table 1. Briefly, the mean age was 64.4 ± 13.2 years, median body mass index (BMI) was 28.2 kg/m2 (interquartile range [IQR] 25.0 – 34.2), and the proportion of women was 33%. Common comorbidities included diabetes mellitus (23% of patients), heart failure (29%), and chronic obstructive pulmonary disease (COPD, 14%). The most common procedures were isolated valve surgery (36%) and isolated coronary artery bypass grafting (33%); 91% of patients underwent sternotomy; and 19% of patients had previously undergone cardiac surgery.
Table 1.
Patient and operative characteristicsa
| C. diff (N = 50) |
No C. diff (N = 5108) |
Overall (N = 5158) |
Hazard Ratio |
P-valueb | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 64.5 (13.4) | 64.4 (13.2) | 64.4 (13.2) | 1.001 | 0.9533 |
| Male | 36 (72.0) | 3414 (66.8) | 3450 (66.9) | 1.264 | 0.4577 |
| Race | 0.0581 | ||||
| White | 37 (74.0) | 4285 (83.9) | 4322 (83.8) | 0.420 | |
| Black | 11 (22.0) | 529 (10.4) | 540 (10.5) | ||
| Other | 2 (4.0) | 294 (5.8) | 296 (5.7) | 0.332 | |
| BMI | 28.8 (25.0, 34.2) | 28.2 (25.1, 32.2) | 28.2 (25.1, 32.3) | 1.003 | 0.8847 |
| Baseline Laboratories | |||||
| WBC, ×103/ml | 7.0 (5.6, 8.6) | 7.0 (5.7, 8.4) | 7.0 (5.7, 8.4) | 0.991 | 0.8799 |
| Creatinine, mg/dL | 1.2 (1.0, 1.6) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.186 | 0.0027 |
| Hemoglobin, g/dL | 13.1 (11.0, 14.4) | 13.4 (12.0, 14.5) | 13.4 (12.0, 14.5) | 0.890 | 0.1161 |
| Cardiac morbidity | |||||
| Heart failure | 23 (46.0) | 1482 (29.0) | 1505 (29.2) | 2.093 | 0.0092 |
| Ejection fraction | 50.0 (35.0, 55.0) | 55.0 (48.0, 60.0) | 55.0 (48.0, 60.0) | 0.962 | <.0001 |
| Previous cardiac surgery | 16 (32.0) | 942 (18.4) | 958 (18.6) | 2.071 | 0.0163 |
| Baseline circulatory support | 3 (6.0) | 132 (2.6) | 135 (2.6) | 2.383 | 0.1447 |
| History of infective endocarditis | 61 (1.2) | 61 (1.2) | 0.000 | 0.9817 | |
| Noncardiac morbidity | |||||
| Diabetesc | 12 (24.0) | 1157 (22.7) | 1169 (22.7) | 1.084 | 0.8081 |
| COPD | 0.1895 | ||||
| None | 38 (76.0) | 4374 (85.6) | 4412 (85.5) | ||
| Mild or moderate | 10 (20.0) | 634 (12.4) | 644 (12.5) | 1.814 | |
| Severe | 2 (4.0) | 100 (2.0) | 102 (2.0) | 2.303 | |
| Renal Failure | 0.0008 | ||||
| No | 36 (72.0) | 4526 (88.6) | 4562 (88.4) | ||
| Yes, Dialysis Dependent | 5 (10.0) | 85 (1.7) | 90 (1.7) | 2.309 | |
| Yes, Not Dialysis | 9 (18.0) | 497 (9.7) | 506 (9.8) | 7.428 | |
| Dependent | |||||
| History of cerebrovascular accident | 7 (14.0) | 513 (10.0) | 520 (10.1) | 1.458 | 0.3551 |
| Operative | |||||
| Surgery time, hours | 5.1 (4.2, 6.6) | 4.2 (3.3, 5.2) | 4.2 (3.3, 5.2) | 1.458 | <.0001 |
| Bypass time, hoursd | 2.1 (1.6, 3.1) | 1.8 (1.3, 2.3) | 1.8 (1.3, 2.3) | 1.523 | 0.0011 |
| Sternotomy | 48 (96.0) | 4621 (90.5) | 4669 (90.5) | 2.512 | 0.2018 |
| Surgery Type | 0.4590 | ||||
| Elective | 33 (66.0) | 3773 (73.9) | 3806 (73.8) | ||
| Urgent | 15 (30.0) | 1199 (23.5) | 1214 (23.5) | 1.430 | |
| Emergent | 2 (4.0) | 136 (2.7) | 138 (2.7) | 1.681 | |
| Procedure | <.0001 | ||||
| Isolated CABG | 9 (18.0) | 1668 (32.7) | 1677 (32.5) | 0.407 | |
| Isolated valve | 12 (24.0) | 1866 (36.5) | 1878 (36.4) | 0.487 | |
| CABG + valve | 9 (18.0) | 683 (13.4) | 692 (13.4) | ||
| Transplant or VAD | 10 (20.0) | 112 (2.2) | 122 (2.4) | 6.499 | |
| Thoracic aortic | 6 (12.0) | 422 (8.3) | 428 (8.3) | 1.080 | |
| Other | 4 (8.0) | 357 (7.0) | 361 (7.0) | 0.847 | |
| Other | |||||
| Transferred from outside ospital | 4 (8.0) | 717 (14.0) | 721 (14.0) | 0.535 | 0.2302 |
| Pre-operative antibiotic prophylaxis | 0.0344 | ||||
| 1st generation cephalosporin | 23 (46.0) | 1836 (36.0) | 1859 (36.1) | 0.922 | |
| 2nd generation cephalosporin | 13 (26.0) | 2232 (43.7) | 2245 (43.6) | 0.431 | |
| Other | 14 (28.0) | 1036 (20.3) | 1050 (20.4) | ||
| Days admitted prior to surgery | 0.0 (0.0, 4.0) | 0.0 (0.0, 2.0) | 0.0 (0.0, 2.0) | 1.008 | 0.7761 |
Continuous variables are expressed as mean (SD) or median (IQR) and categorical variables as count (%).
Based on Cox proportional hazards model where outcome is time to C. diff infection and predictor is patient or operative characteristic.
Insulin or oral medications.
91.1% of patients had on-pump surgical procedures.
Key: CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; SD, standard deviation; VAD, ventricular assist device; WBC, white blood cells
CDI was the third most common infection observed (0.97%) after pneumonia (2.38%) and blood stream infections (1.09%). Compared to patients without CDI, patients with CDI were more likely to have higher creatinine, lower ejection fractions, prior cardiac surgery, and renal failure (with or without dialysis). Median surgery and bypass times were longer for patients with CDI, and patients with CDI were more likely to have undergone combined procedures, ventricular assist device (VAD) placement or replacement, or heart transplant than patients without CDI. Patients with CDI were also more likely to have received more than 48 hours of post-operative antibiotic prophylaxis; were less likely to have received 2nd generation cephalosporins as the post-operative antibiotic prophylaxis; and were more likely to have longer times in the ICU and on ventilation (Table 2). Post-operative hyperglycemic episodes and infections other than CDI were also more common in patients with CDI.
Table 2.
Postoperative characteristicsa
| C. diff (N = 50) |
No C. diff (N = 5108) |
Overall (N = 5158) |
Hazard Ratio |
P-valueb | |
|---|---|---|---|---|---|
| Days of post-operative antibiotic prophylaxis | <.0001 | ||||
| 2 Days (24–48 hours) | 13 (26.0) | 2107 (41.2) | 2120 (41.1) | ||
| 1 Day (0–24 hours) | 11 (22.0) | 2585 (50.6) | 2596 (50.3) | 0.692 | |
| 3 Days (>48 hours) | 26 (52.0) | 416 (8.1) | 442 (8.6) | 10.033 | |
| Post-operative antibiotic prophylaxis | 0.0358 | ||||
| 1st generation cephalosporin | 19 (38.0) | 1721 (33.7) | 1740 (33.7) | 0.721 | |
| 2nd generation cephalosporin | 14 (28.0) | 2270 (44.4) | 2284 (44.3) | 0.404 | |
| Other | 17 (34.0) | 1117 (21.9) | 1134 (22.0) | ||
| ICU days | 5.0 (2.0, 8.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 1.069 | <.0001 |
| Ventilation duration, days | 1.0 (0.6, 2.1) | 0.6 (0.4, 1.0) | 0.6 (0.4, 1.0) | 1.087 | 0.0001 |
| Packed red blood cells, unitc | 2.0 (0.0, 8.0) | 0.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 1.225 | <.0001 |
| LOS from surgery (truncated at onset of C. diff), days | 10.0 (7.0, 17.0) | 7.0 (6.0, 10.0) | 7.0 (6.0, 10.0) | 1.069 | <.0001 |
| Non- C. difficile infectiond | 14 (28.0) | 551 (10.8) | 565 (11.0) | 3.180 | 0.0002 |
| Hyperglycemia | 37 (74.0) | 2191 (43.0) | 2228 (43.3) | 3.766 | <.0001 |
Continuous variables are expressed as median (IQR). Categorical variable is described as count (%).
Based on Cox proportional hazards model where outcome is time to C. diff infection and predictor is postoperative characteristic.
48.1% of patients received packed red blood cell transfusions.
Onset of non- C. difficile infection prior to onset of CDI for C. difficile patients.
Frequency, Severity, and Timing of CDI
There were 52 identified CDIs in 50 patients during the 65 day follow up period (0.97%). Two of the patients with CDI (4%) required total colectomy. Median time to CDI onset was 17 days after surgery (IQR, 6–28 days; Figure 2). Onset of CDI occurred prior to hospital discharge in 52% of patients (n = 26) (Table 3); onset of CDI occurred on the day of discharge in two patients. Ten patients had a major infection prior to CDI (20%), and six patients had a minor infection prior to CDI (12%); two patients had both a major and a minor infection prior to onset of CDI.
Figure 2.
Cumulative Incidence of C. difficile Infections
Table 3.
Infection timing for patients with C. diffa
| C. diff (N = 50) |
|
|---|---|
| C. diff Onset | |
| Before index hospital discharge | 26 (52.0) |
| Time to C. diff onset, days | 17.0 (8.0, 30.0) |
| Other Infections | |
| Major infection before C. diff | 10 (20.0) |
| Minor infection before C. diff | 6 (12.0) |
| Any infection (major or minor) before C. diff | 14 (28.0) |
Continuous variable is expressed as median (IQR); categorical variables are expressed as frequency (percent).
Risk Factors Associated with CDI
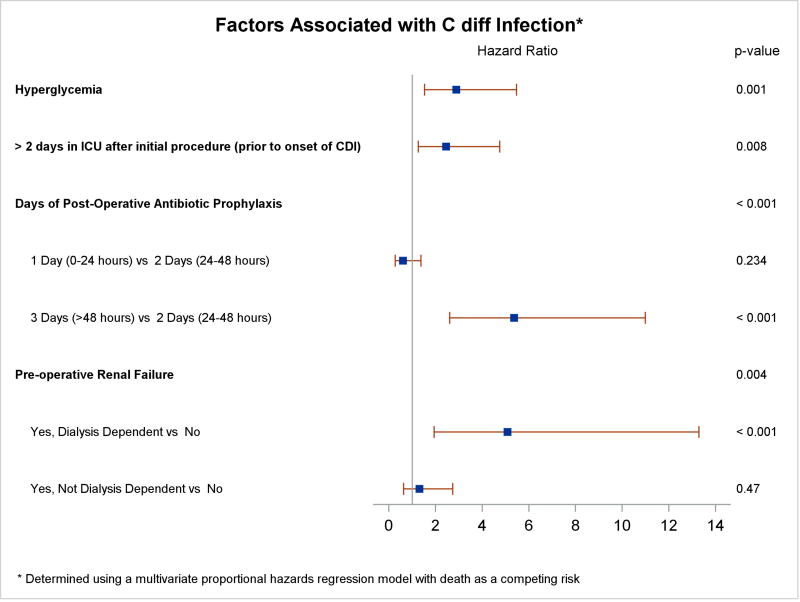
Renal failure was the only preoperative patient characteristic associated with a higher risk of CDI in multivariable analysis (hazard ratio [HR] 5.08 for dialysis-dependent renal failure compared to no renal failure; 95% CI, 1.94 – 13.28; p = 0.004). Approximately half of patients were admitted on the day of surgery (median days admitted prior to surgery 0.0, IQR 0.0 – 2.0); preoperative LOS was not associated with increased risk of CDI in the multivariable model. Operative and postoperative variables associated with greater CDI risk include hyperglycemia (HR 2.89; 95% CI, 1.53 – 5.47; p = 0.001) and days of post-operative antibiotic prophylaxis (HR 5.36 for 3 days versus 2 days; 95% CI, 2.61 – 10.99, p < 0.001). Being in the intensive care unit (ICU) longer than 2 days was also associated with increased risk of CDI (HR 2.45; 95% CI, 1.27 – 4.75, p =0.008, Figure 3). There was no evidence of a differential effect of hyperglycemia by diabetes status (p = 0.327).
Figure 3.
Risk Factors for C. difficile Infections
Length of Stay and Readmissions
For the 26 patients whose onset of CDI occurred during the index hospitalization, the observed mean LOS from hospital admission was 35.3 ± 21.6 days compared to 17.4 ± 12.0 days and 11.0 ± 9.1 days for patients diagnosed with CDI after discharge and for patients without CDI, respectively. A multi-state Markov model was used to estimate the incremental LOS of the index hospitalization following surgery; excess LOS of the index hospitalization due to CDI was 12.29 ± 3.22 days (bootstrap 95% CI, 7.39 – 17.76).
Thirteen patients who acquired CDI during the follow up period were readmitted a total of 15 times in the first 30 days after surgery (0.4343 readmissions per patient month of follow up), whereas 602 patients without CDI were readmitted 642 times in the first 30 days after surgery (0.1310 readmissions per patient month of follow up). Over the entire 65 day follow up period, readmission rates were 0.2255 versus 0.0903 per patient month of follow up for patients with and without CDI, respectively.
CDI and Mortality
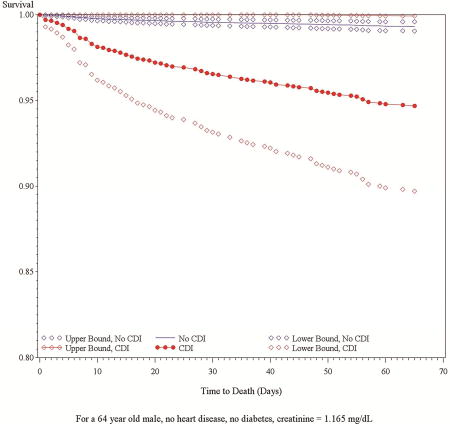
Death occurred more frequently during the 65 day follow up period in patients with CDI than in patients without CDI (10.0% vs. 1.8%, p < 0.001). In a proportional hazards regression model treating CDI as a time-dependent variable, CDI had a substantial impact on survival (hazard ratio [HR]: 5.45; 95% CI: 2.14–13.87). The model included non-C. difficile infection as a time-varying covariate (HR: 4.06; 95% CI: 2.49 – 6.62). The model also adjusted for sex (HR: 0.54 (male); 95% CI: 0.36–0.80), age (HR: 1.04; 95% CI: 1.02–1.05), baseline creatinine (HR: 1.20; 95% CI: 1.09–1.31), CHF (HR: 2.01; 95% CI: 1.34–3.02), diabetes (HR: 1.57; 95% CI: 1.03–2.38) which were previously shown to be predictors of mortality independently of CDI (Table 4).37 The effect of CDI on survival for an average patient in the cohort (male, age 64, creatinine 1.165 mg/dL, no diabetes or heart failure) is shown in the Central Picture.
Table 4.
Impact of CDI on Mortality
| Hazard Ratio | 95% Confidence Interval |
p-value | |
|---|---|---|---|
| CDI statusa | 5.449 | (2.14, 13.87) | 0.0004 |
| Male | 0.535 | (0.36, 0.8) | 0.0023 |
| Age | 1.036 | (1.02, 1.05) | <.0001 |
| Creatinine, per mg/dL ? | 1.199 | (1.09, 1.31) | 0.0001 |
| Diabetes (yes/no) | 1.569 | (1.03, 2.38) | 0.0350 |
| Non-C. difficile infection statusb | 4.063 | (2.49, 6.62) | <.0001 |
| Heart Failure (yes/no) | 2.011 | (1.34, 3.02) | 0.0007 |
CDI status is a time-varying covariate.
Non- C. difficile infection status is a time-varying covariate and refers to infection prior to the onset of CDI.
Central Picture.
Survival by Time-Varying C. difficile Infection Status
DISCUSSION
Most previous studies of major infections in cardiac surgery patients have focused on a subset of infections, predominantly surgical site infections and bloodstream infections35,36, yet a recent large prospective cohort study showed that Clostridium difficile infections (CDI) were the third most common major infection after pneumonia and bloodstream infections.37 Additionally, most of these studies have focused on the first 30 days after surgery and/or the index hospitalization period as this is the timeframe for which national databases such as the Premier Perspective Comparative database, the Nationwide Inpatient Sample database, and the Society of Thoracic Surgeons database collect events.24,35,48
Recent results of an active surveillance study funded by the Centers for Disease Control and Prevention’s Emerging Infections Program showed that while the majority of CDI cases in the United States were related to healthcare exposure (i.e. an inpatient or outpatient visit within 12 weeks prior to the collection of a C. difficile-positive stool sample), only 24% of cases observed had an in-hospital onset.25 The data from the CTSN infection study, therefore, provide a unique opportunity to examine the incidence of CDI in the cardiac surgery population and risk factors associated with its occurrence as well as the relationship between CDI and adverse outcomes.
As would be expected based on the CDC’s surveillance, nearly half of all CDIs (48%) occurred after index hospital discharge, and 25% of all CDIs occurred after day 30 in our study. This may explain why frequency of CDI in this study (50 patients, or 0.97%) was higher than the 0.21–0.75% reported elsewhere from national databases of cardiac surgery patients.24,48,49 CDI was more common than expected during study planning and was, in fact, collected as an “other” serious infection. Diagnosis was therefore not protocolized but determined according to standard clinical care at each site.
Comorbidities have been frequently associated with risk of CDI.1,2,8,9,11,17,24,31,33,34,50 Not surprisingly, we found that patients who developed CDI were more likely to suffer from renal failure pre-operatively and were more likely to have longer ICU stays. Duration of ICU stay and procedure were related --VAD and transplant patients spent longer in the ICU than patients undergoing other procedures. Acute hyperglycemia was associated with an increased risk of CDI. It is unclear, however, if hyperglycemia is indicative of a sicker, more vulnerable patient or if hyperglycemia in and of itself increases the risk of infection, particularly since diabetes was not associated with risk of CDI and we did not observe a differential risk of hyperglycemia by diabetes.
Unlike other studies9,12,13,17, however, we did not find significantly higher incidence with older age, diabetes, increased BMI, or COPD; with urgent or emergent procedure status; in women; or in Caucasian patients. In contrast, African Americans were the racial group that contracted CDI most frequently. Given that only 50 patients in this cohort contracted CDI and the cohort was predominantly male, the analysis may have simply been underpowered to detect a difference. It is also possible that varying diagnostic criteria by site may have obscured any risks associated with demographics. There was not a statistically significant difference in CDI incidence in patients who did not undergo a sternotomy compared to those who did though it’s worth noting that the incidence in both groups is low, so a difference would have to be fairly large to be detectable.
Moreover, other studies have identified additional risk factors for CDI, including malignancy, chemotherapy, intra-abdominal therapy, malnutrition and previous CDI.33,34 As this study was designed to focus on management practices and the risk of infection, many of the variables identified in other studies as risk factors of CDI were not collected in this study.
Although patients with CDI were readmitted more often during the 65 day follow-up period, this appears to be indicative of the fact that hospital exposure and comorbidities increase the risk of CDI as readmissions tended to be for reasons other than CDI; only 7 of the 31 readmissions in this group were due to CDI. In most cases, onset of CDI occurred during index hospitalization or during a readmission hospitalization. Readmissions for CDI occurred in close proximity to a hospital discharge, consistent with the CDC’s surveillance study.
As was observed in other reports1–4,6,8,9,12,17,21,32,50–52, mortality increased substantially with CDI. This observation held after adjusting for age, sex, diabetes, CHF, baseline creatinine, and time-varying non-C. difficile infection (HR = 5.45, 95% CI: 2.14–13.87, p < 0.001). Mortality observed in patients with CDI in this cohort of cardiac surgery patients (10.0%) was comparable to the death rates from healthcare-associated CDI in surgical and non-surgical patients in the US and Canada over the same time period (6–35%).53,54 Despite the fact that patients with CDI tended to generally be at higher risk of adverse outcomes, we did not observe any increase in any major adverse cardiac or cerebrovascular events other than death (MI, CVA, or TIA) at any point during the 65 day follow up. A single CVA occurring after onset of CDI was the only non-fatal reported adverse event among the 50 patients ever diagnosed with CDI.
Risk of CDI increases substantially with prolonged antibiotic exposure as suppression of the bowel flora allows C. difficile bacteria to grow55 ; this effect is thought to be particularly pronounced with second generation cephalosporins.56 Antibiotic prophylaxis lasting 48 hours or less was protective of CDI but antibiotic prophylaxis lasting 3 days or longer was associated with higher risk of CDI regardless of type of antibiotic prophylaxis. We had anticipated that a prior post-operative infection would increase the risk of CDI as the initial post-operative infection would presumably result in antibiotic treatment yet only 28% of first CDI cases occurred after another infection (major or minor), and other (non-C. difficile) infection was not significantly associated with increased risk of CDI.
Though type of antibiotic was significant in univariable analyses with 2nd generation cephalosporins associated with a decreased hazard of CDI, the finding did not hold in a multivariable model, possibly because the type of antibiotic was correlated with the duration the antibiotic was given. This may also be due to the limited size of the study (50 patients with CDI) as a recent analysis of 154,200 cardiac surgery patients in the Premier database showed antibiotic prophylaxis with cephalosporin was associated with decreased CDI risk (though it did not distinguish between 1st and 2nd generation cephalosporins).49 That study also confirmed increased risk of CDI with antibiotic prophylaxis that extended past 48 hours.
Limitations
Despite a cohort of over 5,000 patients, the number of patients with CDI was low, limiting power. CDI was potentially underreported in this study because it was not included in the predefined list of major infections in the protocol; instead, it was captured as a serious “other” infection. Diagnostic criteria were not protocolized, and we do not know how each diagnosis was made (i.e. NAAT vs. other methods). However, the study was designed to capture all post-operative infections, all events were reviewed and adjudicated by an Event Adjudication Committee of infectious disease experts, and follow up through end of study was 98% complete. Given the thoroughness of data capture and the fact that the reported incidence in this study was higher than in other studies,1,2,4,13 we believe the chance of bias due to underreporting is low.
Medications other than prophylactic antibiotics were not captured in the database which precluded analysis of antibiotics administered for other infections that occurred prior to CDI. It also precluded analysis of medications such as proton pump inhibitors that have been previously shown to be risk factors for CDI.48,57 However, other infections, serious or not (before the onset of CDI for patients with CDI), were not associated with an increased risk of CDI.
Discharge disposition was also not captured in the database, precluding analysis to determine if patients discharged to a rehabilitation or nursing facility were at increased risk of post-discharge CDI compared to patients who were discharged home.
Lastly, the excess LOS due to CDI should be interpreted with caution as it does not take into account other potentially informative covariates (e.g. index surgical procedure). As the study was designed to focus on management practices and infection, other complications and adverse events were not collected, and these may have affected LOS overall or in the ICU.
CONCLUSION
CDI was a common infection following cardiac surgery and was associated with significant increases on LOS and mortality. Despite intense efforts to reduce the incidence of healthcare-associated infections, there are still opportunities to improve adherence to quality improvement measures, particularly glycemic control and optimal duration of antibiotic prophylaxis (≤ 48 hours), to reduce the rates of CDI. High-risk patients, such as those with renal failure or undergoing complex procedures such as VAD placement or transplant who will spend an extended length of time in the ICU, are particularly vulnerable to CDI and may warrant additional precautions to reduce the morbidity and mortality associated with CDI. Providers should continue to be vigilant about CDI after discharge as onset is frequently more than two weeks after surgery.
Supplementary Material
Central Message.
Prolonged antibiotic prophylaxis (> 48 hours) was associated with risk of CDI, the third most common major infection after cardiac surgery. CDI was associated with a substantial increase in mortality.
Perspective Statement.
The incidence and severity of CDI has increased recently. Our study found that CDI was associated with longer length of stay, frequent readmissions, and decreased survival. Median time to onset of CDI was > 2 weeks postoperatively with nearly half of patients (48%) first diagnosed after discharge. Limiting antibiotic prophylaxis to 2 days and controlling blood glucose may reduce the incidence of CDI.
Acknowledgments
Sources of Funding: A cooperative agreement (U01 HL088942) funded by the National Heart, Lung, and Blood Institute and the National Institute of Neurological Disorders and Stroke of the NIH and the Canadian Institutes of Health Research.
Abbreviations
- BMI
Body mass index
- CABG
Coronary artery bypass grafting
- CDC
Centers for Disease Control and Prevention
- CDI
C. difficile infection
- CHF
Congestive heart failure
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CTSN
Cardiothoracic Surgery Trials Network
- DCC
Data coordinating center
- EAC
Event adjudication committee
- HR
Hazard ratio
- ICU
Intensive care unit
- IQR
Interquartile range
- LOS
Length of stay
- MI
Myocardial infarction
- NAAT
Nucleic acid amplification test
- SD
Standard deviation
- TIA
Transient ischemic attack
- VAD
Ventricular assist device
- WBC
White blood cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registry Number: NCT01089712
Conflicts of Interest: All authors have no conflicts of interest to disclose.
References
- 1.Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surgical infections. 2007 Dec;8(6):557–566. doi: 10.1089/sur.2006.062. [DOI] [PubMed] [Google Scholar]
- 2.Keshavamurthy S, Koch CG, Fraser TG, et al. Clostridium difficile infection after cardiac surgery: prevalence, morbidity, mortality, and resource utilization. The Journal of thoracic and cardiovascular surgery. 2014 Dec;148(6):3157–3165. e3151–3155. doi: 10.1016/j.jtcvs.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. American journal of surgery. 2008 Sep;196(3):384–388. doi: 10.1016/j.amjsurg.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Lemaire A, Dombrovskiy V, Batsides G, et al. The effect of Clostridium difficile infection on cardiac surgery outcomes. Surgical infections. 2015 Feb;16(1):24–28. doi: 10.1089/sur.2013.097. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. The New England journal of medicine. 2008 Oct 30;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 6.Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009 May;144(5):433–439. doi: 10.1001/archsurg.2009.51. discussion 439–440. [DOI] [PubMed] [Google Scholar]
- 7.Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infectious disease clinics of North America. 2015 Mar;29(1):123–134. doi: 10.1016/j.idc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Crabtree T, Aitchison D, Meyers BF, et al. Clostridium difficile in cardiac surgery: risk factors and impact on postoperative outcome. The Annals of thoracic surgery. 2007 Apr;83(4):1396–1402. doi: 10.1016/j.athoracsur.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 9.Greenstein AJ, Byrn JC, Zhang LP, Swedish KA, Jahn AE, Divino CM. Risk factors for the development of fulminant Clostridium difficile colitis. Surgery. 2008 May;143(5):623–629. doi: 10.1016/j.surg.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Annals of surgery. 2002 Mar;235(3):363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert review of gastroenterology & hepatology. 2010 Aug;4(4):409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 12.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerging infectious diseases. 2007 Sep;13(9):1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007 Jul;142(7):624–631. doi: 10.1001/archsurg.142.7.624. discussion 631. [DOI] [PubMed] [Google Scholar]
- 14.Salazar M, Baskin L, Garey KW, DuPont HL. Clostridium difficile-related death rates in Texas 1999–2005. The Journal of infection. 2009 Nov;59(5):303–307. doi: 10.1016/j.jinf.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005 Sep 24–30;366(9491):1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 16.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile-related hospitalizations and case-fatality rate, United States, 2000–2005. Emerging infectious diseases. 2008 Jun;14(6):929–931. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocanu V, Buth KJ, Johnston LB, Davis I, Hirsch GM, Legare JF. The Importance of Continued Quality Improvement Efforts in Monitoring Hospital-Acquired Infection Rates: A Cardiac Surgery Experience. The Annals of thoracic surgery. 2015 Jun;99(6):2061–2069. doi: 10.1016/j.athoracsur.2014.12.075. [DOI] [PubMed] [Google Scholar]
- 18.Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002 Oct;137(10):1096–1100. doi: 10.1001/archsurg.137.10.1096. [DOI] [PubMed] [Google Scholar]
- 19.Stewart DB, Hollenbeak CS. Clostridium difficile colitis: factors associated with outcome and assessment of mortality at a national level. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011 Sep;15(9):1548–1555. doi: 10.1007/s11605-011-1615-6. [DOI] [PubMed] [Google Scholar]
- 20.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. The New England journal of medicine. 2005 Dec 8;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 21.Elixhauser A, Jhung M. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2006. Clostridium Difficile-Associated Disease in U.S. Hospitals, 1993–2005: Statistical Brief #50. [Google Scholar]
- 22.Inkster T. Antibiotic prophylaxis for cardiac surgery: a shift away from traditional cephalosporins? Journal of cardiothoracic and vascular anesthesia. 2009 Dec;23(6):933–935. doi: 10.1053/j.jvca.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Wysowski DK. Increase in deaths related to enterocolitis due to Clostridium difficile in the United States, 1999–2002. Public Health Rep. 2006 Jul-Aug;121(4):361–362. [PMC free article] [PubMed] [Google Scholar]
- 24.Flagg A, Koch CG, Schiltz N, et al. Analysis of Clostridium difficile infections after cardiac surgery: epidemiologic and economic implications from national data. The Journal of thoracic and cardiovascular surgery. 2014 Nov;148(5):2404–2409. doi: 10.1016/j.jtcvs.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. The New England journal of medicine. 2015 Feb 26;372(9):825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguayo C, Flores R, Levesque S, et al. Rapid spread of Clostridium difficile NAP1/027/ST1 in Chile confirms the emergence of the epidemic strain in Latin America. Epidemiology and infection. 2015 Feb 17;:1–5. doi: 10.1017/S0950268815000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngamskulrungroj P, Sanmee S, Pusathit P, et al. Molecular Epidemiology of Clostridium difficile Infection in a Large Teaching Hospital in Thailand. PloS one. 2015;10(5):e0127026. doi: 10.1371/journal.pone.0127026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nanwa N, Kendzerska T, Krahn M, et al. The economic impact of Clostridium difficile infection: a systematic review. The American journal of gastroenterology. 2015 Apr;110(4):511–519. doi: 10.1038/ajg.2015.48. [DOI] [PubMed] [Google Scholar]
- 29.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012 Aug;55(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC infectious diseases. 2016 Jun 18;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishara J, Peled N, Pitlik S, Samra Z. Mortality of patients with antibiotic-associated diarrhoea: the impact of Clostridium difficile. The Journal of hospital infection. 2008 Apr;68(4):308–314. doi: 10.1016/j.jhin.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 32.Musa SA, Moran C, Thomson SJ, et al. Clostridium difficile-associated disease acquired in the cardiothoracic intensive care unit. Journal of cardiothoracic and vascular anesthesia. 2011 Apr;25(2):263–267. doi: 10.1053/j.jvca.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Khanafer N, Vanhems P, Barbut F, Luxemburger C. Factors associated with Clostridium difficile infection: A nested case-control study in a three year prospective cohort. Anaerobe. 2017 Apr;44:117–123. doi: 10.1016/j.anaerobe.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Wu S, Chen R, et al. Risk factors of Clostridium difficile infections among patients in a university hospital in Shanghai, China. Anaerobe. 2014 Dec;30:65–69. doi: 10.1016/j.anaerobe.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Fowler VG, Jr, O'Brien SM, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005 Aug 30;112(9 Suppl):I358–365. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 36.Abboud CS, Wey SB, Baltar VT. Risk factors for mediastinitis after cardiac surgery. The Annals of thoracic surgery. 2004 Feb;77(2):676–683. doi: 10.1016/S0003-4975(03)01523-6. [DOI] [PubMed] [Google Scholar]
- 37.Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol. 2014 Jul 29;64(4):372–381. doi: 10.1016/j.jacc.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American journal of infection control. 2008 Jun;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Ailawadi G, Chang HL, O'Gara PT, et al. Pneumonia after cardiac surgery: Experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. The Journal of thoracic and cardiovascular surgery. 2017 Jun;153(6):1384–1391. e1383. doi: 10.1016/j.jtcvs.2016.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perrault KAK Louis P, Chang Helena L, Mullen John C, Gulack Brian C, Argenziano Michael, Gelijns Annetine C, Ghanta Ravi K, Whitson Bryan A, Williams Deborah L, Sledz-Joyce Nancy, Lima Brian, Greco Giampaolo, Fumakia Nishit, Rose Eric A, Puskas John D, Blackstone Eugene H, Weisel Richard D, Bowdish Michael E. A Prospective Multi-Institutional Cohort Study of Mediastinal Infections after Cardiac Surgery. [accepted June 2017];The Annals of thoracic surgery. doi: 10.1016/j.athoracsur.2017.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine JPGR. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 42.Zhou B, Fine J, Laird G. Goodness-of-fit test for proportional subdistribution hazards model. Stat Med. 2013 Sep 30;32(22):3804–3811. doi: 10.1002/sim.5815. [DOI] [PubMed] [Google Scholar]
- 43.Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2006 Jul-Aug;12(Suppl 3):22–26. doi: 10.4158/EP.12.S3.22. [DOI] [PubMed] [Google Scholar]
- 44.Szekely A, Levin J, Miao Y, et al. Impact of hyperglycemia on perioperative mortality after coronary artery bypass graft surgery. The Journal of thoracic and cardiovascular surgery. 2011 Aug;142(2):430–437. e431. doi: 10.1016/j.jtcvs.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Cardona S, Pasquel FJ, Fayfman M, et al. Hospitalization costs and clinical outcomes in CABG patients treated with intensive insulin therapy. Journal of diabetes and its complications. 2017 Apr;31(4):742–747. doi: 10.1016/j.jdiacomp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Greco G, Ferket BS, D'Alessandro DA, et al. Diabetes and the Association of Postoperative Hyperglycemia With Clinical and Economic Outcomes in Cardiac Surgery. Diabetes care. 2016 Mar;39(3):408–417. doi: 10.2337/dc15-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allignol A, Schumacher M, Beyersmann J. Empirical Transition Matrix of Multi-State Models: The etm Package. J Stat Softw. 2011 Jan;38(4):1–15. [Google Scholar]
- 48.Bateman BT, Rassen JA, Schneeweiss S, et al. Adjuvant vancomycin for antibiotic prophylaxis and risk of Clostridium difficile infection after coronary artery bypass graft surgery. The Journal of thoracic and cardiovascular surgery. 2013 Aug;146(2):472–478. doi: 10.1016/j.jtcvs.2013.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poeran J, Mazumdar M, Rasul R, et al. Antibiotic prophylaxis and risk of Clostridium difficile infection after coronary artery bypass graft surgery. The Journal of thoracic and cardiovascular surgery. 2016 Feb;151(2):589–597. e582. doi: 10.1016/j.jtcvs.2015.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and Clinical Outcomes of Clostridium difficile Infection in the Intensive Care Unit: A Systematic Review and Meta-Analysis. Open forum infectious diseases. 2016 Jan;3(1):ofv186. doi: 10.1093/ofid/ofv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazzeffi M, Gammie J, Taylor B, et al. Healthcare-Associated Infections in Cardiac Surgery Patients With Prolonged Intensive Care Unit Stay. The Annals of thoracic surgery. 2017 Apr;103(4):1165–1170. doi: 10.1016/j.athoracsur.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 52.Bruminhent J, Cawcutt KA, Thongprayoon C, Petterson TM, Kremers WK, Razonable RR. Epidemiology, risk factors, and outcome of Clostridium difficile infection in heart and heart-lung transplant recipients. Clinical transplantation. 2017 Mar 17; doi: 10.1111/ctr.12968. [DOI] [PubMed] [Google Scholar]
- 53.Hota SS, Achonu C, Crowcroft NS, Harvey BJ, Lauwers A, Gardam MA. Determining mortality rates attributable to Clostridium difficile infection. Emerging infectious diseases. 2012 Feb;18(2):305–307. doi: 10.3201/eid1802.101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CDC. Nearly half a million Americons suffered from Clostridium difficile infections in a single year. [Accessed June 25, 2015];CDC Newsroom Release. 2015 [Google Scholar]
- 55.Gorbach SL. Antibiotics and Clostridium difficile. The New England journal of medicine. 1999 Nov 25;341(22):1690–1691. doi: 10.1056/NEJM199911253412211. [DOI] [PubMed] [Google Scholar]
- 56.Nelson DE, Auerbach SB, Baltch AL, et al. Epidemic Clostridium difficile-associated diarrhea: role of second- and third-generation cephalosporins. Infection control and hospital epidemiology. 1994 Feb;15(2):88–94. doi: 10.1086/646867. [DOI] [PubMed] [Google Scholar]
- 57.Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. The American journal of gastroenterology. 2008 Sep;103(9):2308–2313. doi: 10.1111/j.1572-0241.2008.01975.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.