Abstract
Background
Prenatal cocaine exposure (PCE) is linked to addiction and obesity vulnerability. Neural responses to stressful and appetitive cues in adolescents with PCE versus those without have been differentially linked to substance-use initiation. However, no prior studies have assessed cue-reactivity responses among PCE adolescents using a connectivity-based approach.
Methods
Twenty-two PCE and 22 non-prenatally drug-exposed (NDE) age-, sex-, IQ- and BMI-matched adolescents participated in individualized guided imagery with appetitive (favorite-food), stressful and neutral-relaxing cue scripts during functional magnetic resonance imaging. Subjective favorite-food craving scores were collected before and after script exposure. A data-driven voxel-wise intrinsic connectivity distribution analysis was used to identify between-group differences and examine relationships with craving scores.
Results
A group-by-cue interaction effect identified a parietal lobe cluster where PCE versus NDE adolescents showed less connectivity during stressful and more connectivity during neutral-relaxing conditions. Follow-up seed-based connectivity analyses revealed that, among PCE adolescents, the parietal seed was positively connected to inferior parietal and sensory areas and negatively connected to corticolimbic during both stress and neutral-relaxing conditions. For NDE, greater parietal connectivity to parietal, cingulate and sensory areas and lesser parietal connectivity to medial prefrontal areas were found during stress compared to neutral-relaxing cueing. Craving scores inversely correlated with corticolimbic connectivity in PCE, but not NDE adolescents, during the favorite-food condition.
Conclusions
Findings from this first data-driven intrinsic connectivity analysis of PCE influences on adolescent brain function indicate differences relating to PCE status and craving. These findings provide insight into the developmental impact of in utero drug exposure.
Keywords: prenatal cocaine exposure, functional connectivity, appetitive, stress, cue reactivity, craving
1. Introduction
Adolescents prenatally exposed to cocaine as compared with non-drug exposed adolescents exhibit more behavioral problems including internalizing and externalizing behaviors, motivational control and attention deficits (Ackerman et al., 2010; Bandstra et al., 2004; Buckingham-Howes et al., 2013; Min et al., 2014). Preclinical and clinical studies have shown that prenatal cocaine exposure (PCE) is also associated with vulnerability to disorders and poor health states including drug addiction (Delaney-Black et al., 2011; Rando et al., 2013) and obesity (Jastreboff et al., 2013; LaGasse et al., 2011). Adolescents appear especially susceptible to the initiation of substance use and weight-related problems (Delaney-Black et al., 2011; LaGasse et al., 2011; Rando et al., 2013; Richardson et al., 2013) as this time period is crucial for cognitive, emotional and brain development (Andersen and Teicher, 2008). However, while PCE effects have been examined in infants and children (Ackerman et al., 2010; Liu and Lester, 2011; Strathearn and Mayes, 2010a), little is known about how PCE relates to brain correlates of stressful and appetitive processes in adolescents.
Previous preclinical and clinical studies have implicated PCE in impacting corticolimbic brain regions including the anterior cingulate (ACC) (Harvey, 2004), prefrontal cortex (PFC) (Grewen et al., 2014; McCarthy and Bhide, 2012; McCarthy et al., 2014), amygdala (Li et al., 2016) and ventral striatum (Harvey et al.; Wang et al., 2013). Dysregulation of corticolimbic pathways have been shown in response to reward-related and stressful cues among individuals with obesity and with cocaine addiction (Jasinska et al., 2014; Jastreboff et al., 2013; Potenza, 2012). Thus, alterations in reward-related brain regions (Haber and Knutson, 2009) may explain some of the detrimental outcomes related to PCE, including difficulties with motivational control (Chambers et al., 2003), stress processing (Andersen and Teicher, 2008; Oberlander et al., 2008), arousal regulation (Mayes, 2002; Mayes et al., 1998) and attention allocation (Ackerman et al., 2010; Berridge and Robinson, 1998; Min et al., 2014). Here, we used functional magnetic resonance imaging (fMRI) to investigate whole-brain connectivity underlying responses to favorite-food and stressful cues in adolescents with and without PCE.
Using an activation-based approach, we recently demonstrated blunted neural activation within corticolimbic regions among PCE compared to NDE adolescents when exposed to personally relevant appetitive (favorite-food) stimuli (Yip et al., 2014). However, this prior study used a general-linear-model based approach, one that has limitations (Xu, 2015), and in the study we did not examine for functional connectivity relating to PCE. Functional connectivity examines synchrony of regional activations over time, identifying patterns of correlated brain activity (Chen and Glover, 2015; Scheinost et al., 2012) and providing a systems-level assessment of brain functionality. To understand more completely the impact of PCE on brain function during exposure to personally relevant stimuli, it is important to understand how functional connectivity during cue exposure may relate to PCE status, and how these changes relate to subjective measures such as craving. This study therefore uses a data-driven approach to examine functional connectivity in PCE adolescents during stressful, appetitive and neutral-relaxing conditions.
Data-driven functional connectivity studies in addiction point towards the involvement of large-scale brain networks including a default mode network (DMN; involving the posterior cingulate (PCC) and medial prefrontal cortices (mPFC)) and salience network (involving the insular and anterior cingulate cortices). Consistent with these findings, data from two recent resting-state studies suggest that PCE adolescents’ functional connectivity is altered in the amygdala and DMN, compared to NDE counterparts (Li et al., 2016; Li et al., 2011). However, to our knowledge, no prior studies have assessed functional connectivity amongst PCE adolescents during the processing of appetitive, stressful and neutral-relaxing stimuli.
Here, we employ a recently described data-driven voxel-wise connectivity analysis, intrinsic connectivity distribution (ICD) (Scheinost et al., 2012), to identify between-group differences and correlations with self-reported food-craving ratings. Unlike some other functional connectivity analytic approaches, ICD is data-driven and does not require the definition of an arbitrary threshold or regions of interest and thus is model-free and unbiased. While ICD does not require hypotheses, given previous findings, we hypothesized that ICD would identify connectivity differences in corticolimbic (Harvey, 2004; McCarthy and Bhide, 2012; McCarthy et al., 2014) and DMN (Li et al., 2013; Li et al., 2011) regions during stressful and appetitive processing between the PCE and NDE groups, as well as reveal less well-characterized regions and networks. We also hypothesized that food-craving scores would be inversely correlated with the degree of connectivity in these regions during the stressful and appetitive conditions.
2. Material and Methods
2.1 Participants
Twenty-two PCE adolescents (8 female) and 22 age-, gender-, race/ethnicity-, BMI- and IQ-matched NDE healthy adolescents (8 female) aged 14 to 17 years participated in this study (Table 1). Exclusion criteria included current diagnosis of Axis I neuropsychiatric disorders as determined by the National Institute of Health Diagnostic Interview Schedule for Children (C-DISC-4.0-Y) (Shaffer et al., 2000). Fifteen PCE and seven NDE adolescents reported use of drugs such as alcohol, cigarettes and cannabis. Groups did not differ in reported substance use (Table 1). Other exclusion criteria included any contraindication for magnetic resonance imaging.
Table 1.
| Demographic | PCE group (N=22) | NDE group (N=22) | PCE vs. NDE P value | ||
|---|---|---|---|---|---|
| Mean | SE | Mean | SE | P | |
| Age | 14.91 | 0.25 | 14.50 | 0.13 | 0.160 |
| Kaufman IQ | 90.53 | 2.96 | 99.20 | 3.36 | 0.061 |
| BMI | 25.27 | 0.94 | 25.95 | 1.39 | 0.687 |
| N | % | N | % | ||
| Gender | |||||
| Male | 14 | 63.60% | 14 | 63.60% | 0.999 |
| Female | 8 | 36.40% | 8 | 36.40% | |
| Race/Ethnicity | |||||
| African American | 18 | 81.8% | 13 | 59.1% | 0.535 |
| Caucasian | 2 | 9.1% | 3 | 13.6% | |
| Asian | 1 | 4.5% | 2 | 9.1% | |
| Hispanic | 1 | 4.5% | 0 | 0.0% | |
| Biracial | 0 | 0.0% | 1 | 4.5% | |
| Lifetime Substance Use | |||||
| Alcohol | 11 | 50.0% | 6 | 27.3% | 0.215 |
| Cigarettes | 9 | 40.9% | 5 | 22.7% | 0.332 |
| Cannabis | 10 | 45.5% | 4 | 18.2% | 0.104 |
| Cocaine | 1 | 4.5% | 0 | 0.0% | 0.999 |
| Opiates | 2 | 9.1% | 0 | 0.0% | 0.488 |
| Methamphetamine | 1 | 4.5% | 0 | 0.0% | 0.999 |
| Inhalants | 1 | 4.5% | 0 | 0.0% | 0.999 |
| Steroids | 2 | 9.1% | 0 | 0.0% | 0.488 |
Abbreviations: PCE = Prenatal Cocaine Exposed Adolescents, NDE = Non-Drug Exposed Adolescents, SE = Standard Error, PTSD = Post Traumatic Stress Disorder
There are no-between group differences between the PCE and NDE groups.
No LSD, Ecstasy or PCP use was reported by either group.
Adolescents were recruited from a larger pool of PCE and NDE participants that have been followed since birth (Hommer et al., 2013; Jastreboff et al., 2013; Mayes et al., 2005; Rando et al., 2013; Yip et al., 2014). Cocaine-using mothers were recruited based on self-report and a positive urine toxicology test during pregnancy or immediately after birth. All study procedures were approved by the Human Investigation Committee of the Yale University School of Medicine, and all participants signed a written informed consent. Task-based fMRI activation results on this sample has been published previously (Yip et al., 2014); however, differences in functional connectivity were not assessed previously.
2.2 Imagery Task
All adolescents participated in a well-validated imagery task (Chaplin et al., 2014; Hommer et al., 2013; Jastreboff et al., 2013; Miller et al., 1987; Potenza, 2012; Sinha, 2009) composed of 2-minute individualized imagery scripts of appetitive, stressful or neutral-relaxing content. Each script was based on a personal experience described by participants, developed using the scene development questionnaire (Sinha, 2009) and audiotaped by a clinician for presentation during the fMRI session. All scripts were standardized to ensure style, length and content uniformity. All scripts were subjectively rated an 8 out of 10 or higher on a Likert scale of their most pleasurable, stressful and relaxing experiences. Examples of appetitive, stressful and neutral-relaxing situations included eating pizza or ice cream, conflicts with significant others or family problems, and sitting in the park or relaxing in one’s room, respectively. Subjective ratings of craving were collected immediately before and after each script. Participants were asked, “Imagine you have [participant’s favorite food] in front of you right now; how much do you want [participant’s favorite food] right now?” Participants responded verbally using a Likert scale from 0 to 10, where 0 represents “not at all” and 10 represents “more than ever.” PCE and NDE groups did not differ in their subjective ratings of hunger before (p=0.18) and after the fMRI scan (p=0.38).
Six script trials (2 per condition) were presented using a block design during fMRI scanning. Script order was randomized and counterbalanced across subjects. The same condition was not presented consecutively. Each script was presented only once for each subject. Each trial was comprised of a 5-minute block, including a 1.5-minute quiet baseline period, a 2-minute script audiotaped active imagery period, a 0.5-minute quiet passive imagery period post-script, and 1-minute quiet recovery period. During baseline, participants were instructed not to engage in any specific mental activity and lie still in the scanner. During script imagery, participants were instructed to mentally and physically engage in the scripts based on prior out-of-scanner training. During the recovery period between each script, participants remained still in the scanner until their reported craving matched the craving rating reported at baseline (See Figure S1).. Because there were 2 scripts per condition, craving scores were averaged for each condition to obtain pre- and post-script craving measures.
2.3 Image Acquisition
Due to an equipment upgrade, participants were scanned on two Siemens 3T Tim Trio scanners (Siemens AG, Erlangen, Germany) with identical acquisition procedure and sequences on both magnets. Fourteen PCE and fourteen NDE were scanned on one magnet and the remaining participants were scanned on the other magnet. For each subject, structural data were acquired using a sagittal high-resolution T1-weighted 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (repetition time (TR) = 2530 ms, echo time (TE) = 3.34 ms, flip angle (FA) = 7°, field of view (FOV) = 256×256 mm, matrix=256×256, slice thickness = 1mm3). Anatomical data were acquired in 2D images with same slice locations as the functional data parallel to the anterior-posterior commissure line were acquired (TR = 300 ms, TE=2.5 ms, FA=60°, FOV=220×220 mm, matrix = 256×256, slices =32, slice thickness = 4mm). Functional data were collected using a T2*-sensitive single shot echo-planar pulse sequence (TR = 2000 ms, TE = 25 ms, FA = 85°, FOV = 220×220 mm, matrix = 64×64, slices = 32, slice thickness = 4mm). The first ten volumes of each functional timeseries were discarded to allow the signal to reach a steady state.
2.4 Image Preprocessing
Images were slice-time and motion corrected using SPM5 (http://www.fil.ion.ucl.ac.uk). Trials with linear motion of ± 1.5mm or with a rotation of >2° were excluded. All further analysis was performed using BioImage Suite (Joshi et al., 2011) unless otherwise specified. Several covariates of no interest were regressed out from the data including linear and quadratic drift, six rigid-body motion parameters, mean cerebral-spinal fluid (CSF) signal, mean white matter signal, and mean global signal. A gray-matter mask was applied so that only voxels in the gray matter were used in the analysis.
Consistent with prior studies (Garrison et al., 2016; Zakiniaeiz et al., 2017), connectivity analysis focused on the 2.5-minute imagery period (2-minute script and 0.5-minute quiet imagery), where the same cue trial imagery periods were concatenated for a total of approximately 5 minutes of data for each cue state.
2.5 Voxel-wise Connectivity Analysis
For each participant, whole-brain connectivity at each voxel was measured by ICD as previously described (Scheinost et al., 2012). ICD is similar to other voxel-based connectivity measures (Rubinov and Sporns, 2010) in that it correlates the time course for one voxel to all other timecourses in the brain, a network measure described as degree. These correlations are summarized with a network theory metric and compared to investigate differences between groups and conditions. Particular to ICD, voxel-to-voxel connections are modeled across the entire range of correlation strength. By capturing the distribution of each voxel’s connectivity across the entire brain, the need to define an arbitrary threshold of connection strength is eliminated. For each voxel, this distribution is converted to a survival function that is fitted with a stretched exponential of unknown variance, alpha (α). A larger alpha corresponds to a voxel with a greater number of high correlation connections whereas a smaller alpha corresponds to a lesser number of high correlation connections. Alpha determines the spread of the distribution for each voxel. A parametric image is created with alpha values at each voxel. This process is repeated for all gray-matter voxels to create a parametric image for each participant.
Additionally, to examine relative differences in connectivity, each subject’s map was normalized to the mean by subtracting that subject’s mean across all voxels and dividing by the standard deviation across all voxels to be used in between-group analyses. In healthy individuals this z-score-like normalization does not change the underlying connectivity pattern but allows for investigation of relative differences in connectivity in the presences of large global differences connectivity.
2.6 Seed Connectivity Analysis
Whole-brain ICD connectivity analyses were used to identify main effects and interactions. Follow-up “seed” analyses were based on the regions identified in the whole-brain ICD analysis in order to investigate connectivity patterns related to the regions driving the ICD differences. Timecourses for each voxel in the seed were created. These timecourses were correlated with the timecourse of every other gray-matter voxel in the brain. One map for each participant was created with the Fisher transformed r-values from the correlation representing the correlation strength at each voxel of the seed region.
2.7 Common Space Registration
ICD images were smoothed using a 6-mm Gaussian kernel and were warped to common space through linear and non-linear registrations followed by concatenation of these series. Functional series were linearly registered to the 2D anatomical images. 2D anatomical images were linearly registered to the 3D images. 3D images were non-linearly registered to the template brain. All transformation pairs were calculated independently and combined into a single transformation to reduce interpolation error using BioImage Suite.
2.8 Statistical Analyses
Group-level two tailed t-tests were used to compare groups (PCE and NDE) and conditions (favorite-food, stress and neutral) in ICD connectivity. Results are shown at p<0.05 cluster-level and family-wise error (FWE) corrected as determined by AFNI’s AlphaSim. To examine the effects of an interaction, the degree of connectivity within the region was extracted for each participant under each condition. Post-hoc pairwise comparisons using unpaired-samples t-tests (two-tailed) were conducted between groups and across conditions to understand the nature of the interaction. The Yale Brodmann Atlas was used to localize anatomical brain regions. Additionally, whole-brain correlations of ICD connectivity and subjective craving were conducted using BioImage Suite and were FWE-corrected for multiple comparisons.
3. Results
3.1 Participants
PCE and NDE adolescents groups did not differ in age, gender, race/ethnicity, IQ or BMI. Groups also did not differ in reported substance use of alcohol, tobacco, cannabis, cocaine, opiates, methamphetamine, inhalants or steroids (Table 1). While the PCE and NDE groups were not statistically different in IQ, the NDE group IQ scores were almost 10 points higher than those in the PCE group. IQ was included as a covariate in our connectivity analyses.
Overall, subjective food-craving ratings following the stress condition were higher for PCE individuals (M=6.06, SD=2.93) compared to NDE individuals (M=3.68, SD=3.01), t(42)=2.63, p=0.012, two-tailed. Similarly, subjective food-craving scores following the neutral-relaxing condition were higher for PCE individuals (M=6.13, SD=2.80) compared to NDE individuals (M=4.05, SD=3.19), t(42)=2.27, p=0.028, two-tailed. Subjective food-craving scores following the favorite-food condition did not differ between PCE (M=6.25, SD=2.96) and NDE individuals (M=5.34, SD=3.19), t(42)=0.98, p=0.333, two-tailed. Other behavioral results from this study have been previously published (Yip et al., 2014).
3.2 ICD Connectivity Group Differences
T-tests evaluating the ICD relationships between PCE status and condition revealed no main effects of group or condition. We used the neutral-relaxing condition as an active control for the stressful and favorite-food conditions and compared ICD connectivity between groups. An interaction of group-by-condition implicated a parietal region involving the PCC, precuneus and superior parietal lobe (SPL), t(42)=5.00, p<0.001, (Figure 1) during the stress vs. neutral-relaxing conditions. When IQ was included as a covariate, a similar but smaller parietal region encompassing PCC, precuneus and SPL regions was observed (Figure 2). Follow-up pairwise analyses indicated that the PCE group showed less parietal connectivity during the stress condition when compared to the NDE group, t(42)=2.87, p=0.006. The PCE group also showed greater parietal connectivity during the neutral-relaxing condition when compared to the NDE group, t(42)=3.46, p=0.002, (Figure 1). Differences were not observed in favorite-food compared to neutral-relaxing conditions.
Figure 1. ICD Connectivity Interaction of PCE Status and Condition (Stressful and Neutral-Relaxing).
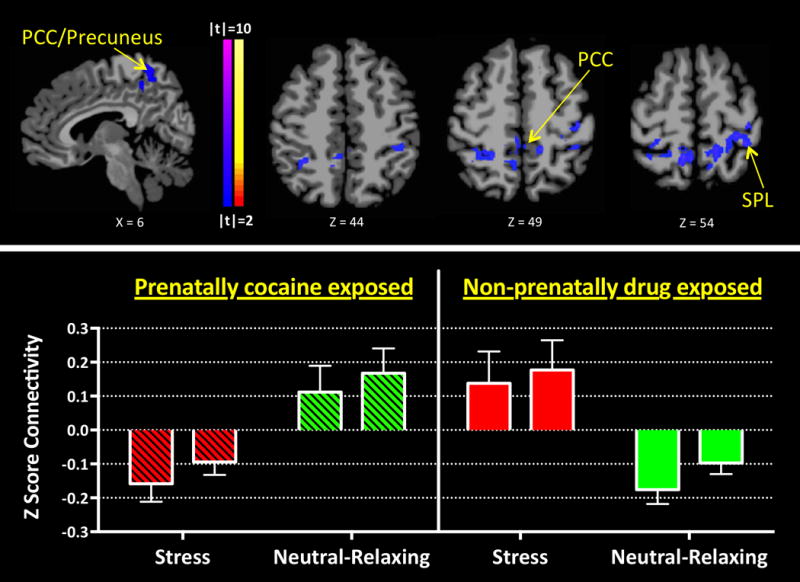
An interaction was observed between stressful and neutral-relaxing conditions and PCE and NDE groups in a parietal region that includes the PCC, precuneus and SPL [t(42)=5.00, p<0.001]. PCE and NDE groups show opposing patterns of connectivity when processing stressful and neutral-relaxing stimuli. The PCE group shows relatively decreased parietal connectivity when processing stressful cues while the NDE group shows relatively increased connectivity. The PCE group also shows an increase in parietal region connectivity when processing neutral-relaxing cues while the NDE group shows a decrease. Left and right bars indicate extracted ICD connectivity values for left and right hemispheres, respectively.
Figure 2. Seed Connectivity Interaction of PCE Status (PCE and NDE) and Condition (Stressful and Neutral-Relaxing) with IQ covariate.
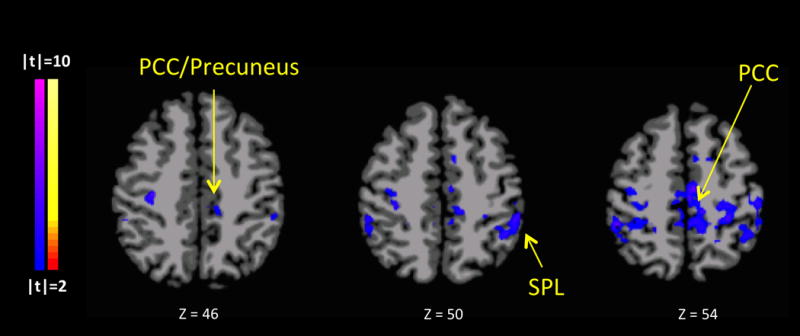
The analysis identified an interaction of group (PCE vs. NDE) by condition (stress vs. neutral) similar (but slightly smaller) than the previously identified parietal region in Figure 1, t(42)=2.04, p<0.05. Total cluster volume for the original parietal cluster is 20,705mm3 (Figure 1), and 14,231mm3 for the IQ-covaried parietal cluster. Similar to the pairwise comparisons of the original (non-covaried) parietal cluster, when stress-cued compared to neutral-relaxing-cued, the PCE group shows relatively decreased parietal connectivity while the NDE group shows relatively increased connectivity.
Follow-up seed-based connectivity analysis using the ICD-identified parietal region (shown in Figure 1) as a seed to the whole brain, identified functionally connected regions, t(42)=2.08, p=0.044. Among the PCE group the parietal seed was positively connected to the inferior parietal lobe (IPL), PCC, angular gyrus, and primary sensory areas and negatively connected to orbitofrontal cortex (OFC), ACC, ventral tegmental area (VTA), ventrolateral PFC (vlPFC), insula, thalamus, caudate, hypothalamus, and cerebellum, during both stress and neutral-relaxing cueing (p<0.05). Among the NDE group, during stress-cueing, the parietal seed was positively connected to the middle cingulate cortex (mCC), IPL, insula, fusiform, dorsolateral PFC (dlPFC), motor, visual and sensory areas and negatively connected to PCC, angular gyrus, inferior frontal gyrus (IFG), OFC, ACC, hippocampus, caudate, thalamus and cerebellum (p<0.05). During neutral-relaxing cueing, the parietal seed was positively connected to the PCC, IPL, insula, fusiform, visual, auditory and sensory areas and negatively connected to the ACC, OFC, hippocampus, caudate, thalamus, and cerebellum (p<0.05) (Figure 3).
Figure 3. Parietal Seed Connectivity by PCE/NDE Status During Stressful and Neutral-Relaxing Conditions.
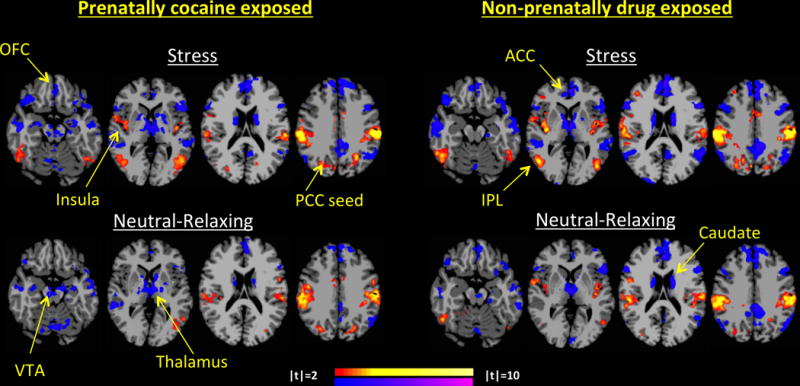
Seeding the parietal region from Figure 1, the PCE group shows increased parietal connectivity to the IPL, PCC, angular gyrus, and primary sensory areas and decreased parietal seed connectivity to OFC, ACC, VTA, vlPFC, insula, thalamus, caudate, hypothalamus, and cerebellum, during both stressful and neutral-relaxing cueing (p<0.05). During stress-cueing, the NDE group shows increased parietal connectivity to the mCC, IPL, insula, fusiform, dlPFC, motor, visual and sensory areas and decreased connectivity to PCC, angular gyrus, IFG, OFC, ACC, hippocampus, caudate, thalamus and cerebellum (p<0.05). During neutral-relaxing cueing, NDE group also shows increased parietal connectivity to the PCC, IPL, insula, fusiform, visual, auditory and sensory areas and decreased connectivity to the ACC, OFC, hippocampus, caudate, thalamus and cerebellum (p<0.05). From left to right, slices Z=-18, Z=2, Z=18 and Z=34 are shown respectively per group and condition.
In order to identify differences in parietal-seed-to-whole-brain connectivity, stress and neutral-relaxing cueing conditions were compared for each group. For NDE adolescents, greater parietal connectivity to IPL, mCC, PCC and sensory areas and lesser parietal connectivity to mPFC and dorsal ACC (dACC) were found during stress compared to neutral-relaxing cueing (p<0.05) (Figure 4). No differences were observed among PCE adolescents.
Figure 4. Parietal Seed Connectivity Differs for Stressful versus Neutral-relaxing in NDE but not PCE adolescents.
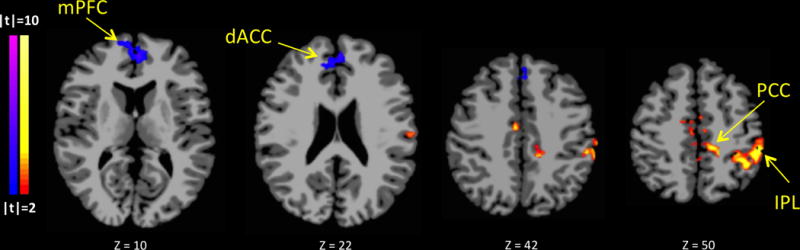
Comparison of stressful vs. neutral-relaxing parietal seed connectivity maps revealed no differences between conditions for PCE adolescents. For NDE adolescents, greater parietal connectivity to IPL, mCC, PCC and sensory areas and lesser parietal connectivity to mPFC and dACC were found during stressful compared to neutral-relaxing cueing (p<0.05).
Subjective Ratings of Craving
ICD identified two clusters that were related to subjective food craving ratings in PCE adolescents following favorite-food and neutral-relaxing conditions. Following favorite-food conditions, food craving scores in PCE adolescents inversely correlated with ventromedial prefrontal cortex (vmPFC)/OFC whole-brain connectivity, and ACC whole-brain connectivity, r(21)=0.78, p<0.001. Following neutral-relaxing conditions, food craving scores in PCE adolescents inversely correlated with connectivity in corticolimbic regions including the right amygdala and bilateral OFC, parahippocampal gryus (PHG) and temporal pole, r(21)=0.78, p<0.001 (Figure 5). No correlations with craving were found in the NDE group.
Figure 5. ICD Connectivity Correlation with Subjective Food Craving in PCE, but not NDE Adolescents.
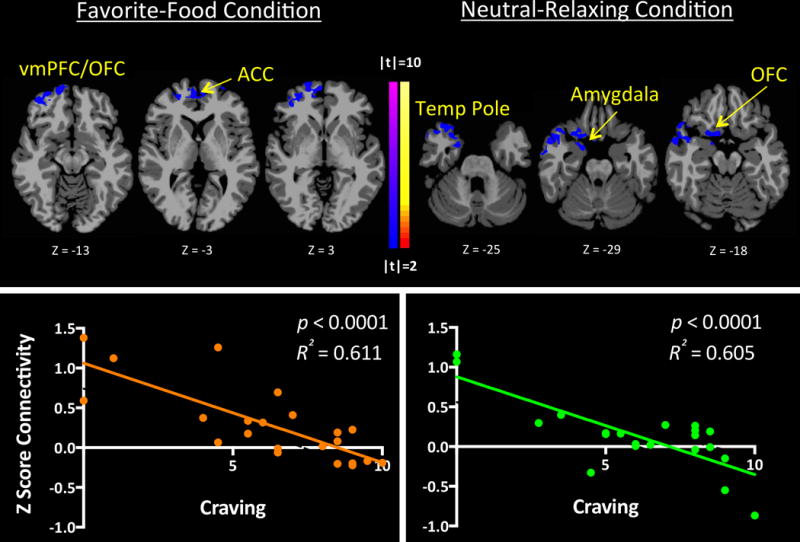
Subjective post-imagery food craving scores correlated inversely with: 1) corticolimbic (vmPFC/OFC and ACC) connectivity during favorite-food cue exposure in PCE adolescents; and, 2) corticolimbic (involving the amygdala, PHG, OFC and temporal pole) connectivity during neutral-relaxing cue exposure in PCE adolescents [r(1,22)=0.423, p<0.05]. No correlations with food craving were found in the NDE group.
4. Discussion
Using a novel, data-driven voxel-wise connectivity method and a well-validated imagery task, we observed altered connectivity among PCE compared to NDE adolescents during the processing of stressful and neutral-relaxing stimuli. PCE participants showed opposite patterns of parietal connectivity compared to NDE participants, where PCE participants showed less connectivity during stressful conditions and more connectivity during neutral-relaxing conditions and NDE participants showed more connectivity during stressful conditions and less connectivity during neutral-relaxing conditions. Seed-based connectivity using the ICD-identified parietal region revealed different patterns of connectivity to the whole brain in PCE and NDE groups; however, when stress and neutral-relaxing conditions were compared connectivity patterns differed in NDE but not PCE adolescents. We also examined relationships between connectivity findings and subjective food cravings to observe that corticolimbic connectivity inversely correlated with food craving scores among PCE but not NDE participants.
Contrary to our hypotheses, corticolimbic regions were not identified in main or interactive effects of group (PCE, NDE) or condition (favorite-food, stressful, neutral-relaxing) during initial ICD analyses. In an activation-based analysis of the same participants, as well as in previous studies, PCE adolescents showed blunted corticolimbic-striatal activations during appetitive but not stressful cues (Peters et al., 2011; Yip et al., 2014). Although the relationship between task-based fMRI activity and data-driven connectivity is not clearly understood, these distinctions may explain the differences in findings between activity and connectivity-based analyses. The current voxel-wise connectivity relationship is a new and important way to investigate neural mechanisms that should be considered jointly with activation studies. Taken together with previous findings (Yip et al., 2014), these data suggest that, while activation patterns may be altered within corticolimbic regions in PCE adolescents, intrinsic functional connectivity between these regions may not.
Besides corticolimbic areas, the DMN may be altered in drug-exposed or addicted populations (Ma et al., 2011). One study has shown that PCE adolescents have higher DMN activity and connectivity during rest and less DMN activity during task, relative to their NDE counterparts (Li et al., 2011), and these dysregulations may persist into early adulthood (Li et al., 2016). The PCC/precuneus brain region, also observed in the current study, is a major hub in the brain’s DMN. Consistent with previous reports (Li et al., 2011), PCC/precuneus connectivity was higher during the neutral-relaxing condition and lower during the stressful condition. Although the neutral-relaxing condition is not identical to rest, it may share similarities in that little cognitive demand is required. NDE participants showed the opposite pattern of connectivity in this major hub of the DMN, suggesting PCE may compromise intrinsic regulation of the DMN.
Our results suggest that PCC/precuneus connectivity contributes significantly to the neural responses to stressful stimuli in PCE as compared to NDE participants. The precuneus is not only involved in the DMN, but also in the regulation of arousal states (Cavanna and Trimble, 2006). The precuneus has been previously implicated in response to smoking cues in tobacco-smokers (Schacht et al., 2013) and in response to alcohol cues in individuals with alcohol dependence, as well as in individuals with both smoking and alcohol-use disorders, which was moderated by level of drug dependence (Courtney et al., 2014). We did not observe comparable findings when PCE adolescents were exposed to a favorite-food condition, which may reflect differences in the groups being studied and/or the relationships between the groups and the cues.
While the PCC/precuneus node that emerged from the data-driven ICD analysis is a major hub in the DMN, seeded connectivity analyses further characterized regions of the DMN. Most notably, during both stressful and neutral-relaxing conditions, in PCE adolescents, the PCC/precuneus seed showed increased connectivity to DMN, auditory, visual and sensory regions and decreased connectivity to salience-network regions. Previous work has shown that the interactions between salience-network and DMN regions are disrupted in individuals with cocaine dependence (Liang et al., 2015). The lack of differences between both stressful and neutral-relaxing conditions implies that these disruptions may be present across several emotional/cognitive states. These findings are also supported by previous literature showing deficits in auditory and visual attention following prenatal tobacco-smoke exposure (Counotte et al., 2011; Jacobsen et al., 2007), suggesting the possibility of a similar effect in PCE adolescents.
Corticolimbic and striatal regions have been implicated in emotional and motivational processes relating to rewarding stimuli. Consistent with previous literature (Hommer et al., 2013; Yip et al., 2014), favorite-food and neutral-relaxing post-imagery food craving measures inversely correlated with connectivity in corticolimbic brain regions. These alterations appeared specific to the PCE group, suggesting that hypo-connectivity in corticolimbic regions may underlie difficulties in reward modulation in this group. Previous work has also suggested that diminished neural activity in corticolimbic regions during reward anticipation may be a vulnerability marker for the development of addictive disorders (Peters et al., 2011).
This study has several strengths including well-matched groups, successful tracking of participants since birth, a well-validated individualized imagery task, and a novel, voxel-wise data-driven connectivity approach. Further, this study presents the first data-driven approach to identify potential targets for further PCE research in the DMN and corticolimbic reward circuitry. Some of the limitations include a relatively small sample size of matched participants, a largely African-American population, a lack of physiological measures (i.e. heart rate) during script conditions in all participants, and a lack of a physical development maturity measure. Future studies should explore gender-related differences in PCE adolescents, as functional connectivity (Scheinost et al., 2015) and cue-induced reactivity (Seo et al., 2011) differences relating to gender have previously been shown. Our relatively modest sample size prevented us from exploring gender-related differences in this study. Future studies should also investigate resting-state data to examine the extent to which the current findings may relate to resting-state brain organization. While this study largely focused on how PCE status relates to neural connectivity, other psychosocial and environmental factors could have impacted the group comparisons. These factors may have included differences in chronic stress in the womb and during childhood relating to caregiving strategies, malnourishment, and domestic violence (Strathearn and Mayes, 2010b; Swain et al., 2007). Thus, future neuroimaging work incorporating assessment of these and other environmental and psychosocial factors in this population is needed. In addition, future investigations should also compare PCE and NDE differences in adulthood to better understand developmental effects in this population.
5. Conclusions
In this first data-driven connectivity analysis of PCE influences on adolescent brain function, PCE adolescents showed altered connectivity compared to NDE adolescents during the processing of favorite-food, stress and neutral-relaxing stimuli, with regions implicated suggesting differences in default-mode and salience networks. These findings suggest similarities with studies that have previously shown altered default-mode and salience network dynamics with cocaine exposure. Further, PCE craving scores correlated with corticolimbic connectivity, suggesting that these networks may be related to motivational drives that may link to vulnerability to substance-use disorders and obesity.
Supplementary Material
Acknowledgments
The study was supported by the National Institutes of Health; Contract grant numbers: P50 DA016556, P50 DA09241, UL1-DE19586, RL1 AA017539, R01 DA006025, R01 DA017863, K05 DA020091; K01 DA039299; Contract grant sponsor: the Office of Research on Women’s Health, the NIH Roadmap for Medical Research/Common Fund. Y. Zakiniaeiz was supported by the Interdepartmental Neuroscience Training Program at Yale University (T32NS4122813), Gruber Science Foundation Fellowship and the National Science Foundation Graduate Research Fellowship Program (NSF-GRFP). Drs. Potenza and Yip were supported by the National Center on Addiction and Substance Abuse.
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Ironwood, Lundbeck, INSYS, Shire, RiverMend Health, Opiant/Lakelight Therapeutics and Jazz Pharmaceuticals; has received research support from the NIH, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming, and Pfizer pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for legal and gambling entities on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the NIH and other agencies; has edited journals; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Footnotes
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript. The other authors reported no biomedical financial interests or other potential conflicts of interest.
References
- Ackerman JP, Riggins T, Black MM. A Review of the Effects of Prenatal Cocaine Exposure Among School-Aged Children. Pediatrics. 2010;125:554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of Prenatal Cocaine Exposure and Child Language Functioning Through Age Seven Years: A Longitudinal Latent Growth Curve Analysis. Substance use & misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic Review of Prenatal Cocaine Exposure and Adolescent Development. Pediatrics. 2013;131:e1917–e1936. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental Neurocircuitry of Motivation in Adolescence: A Critical Period of Addiction Vulnerability. The American journal of psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TM, Visconti KJ, Molfese PJ, Susman EJ, Klein LC, Sinha R, Mayes LC. Prenatal cocaine exposure differentially affects stress responses in girls and boys: Associations with future substance use. Dev Psychopathol FirstView. 2014:1–18. doi: 10.1017/S0954579414000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Glover GH. Functional Magnetic Resonance Imaging Methods. Neuropsychol Rev. 2015;25:289–313. doi: 10.1007/s11065-015-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer ANM, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, London ED, Ray LA. The Association between Cue-Reactivity in the Precuneus and Level of Dependence on Nicotine and Alcohol. Drug Alcohol Depend. 2014;141:21–26. doi: 10.1016/j.drugalcdep.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Partridge RT, Ager J, Sokol RJ. Prenatal and postnatal cocaine exposure predict teen cocaine use. Neurotoxicol Teratol. 2011;33:110–119. doi: 10.1016/j.ntt.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Sinha R, Lacadie CM, Scheinost D, Jastreboff AM, Constable RT, Potenza MN. Functional Connectivity During Exposure to Favorite-Food, Stress, and Neutral-Relaxing Imagery Differs Between Smokers and Nonsmokers. Nicotine Tob Res. 2016 doi: 10.1093/ntr/ntw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewen K, Burchinal M, Vachet C, Gouttard S, Gilmore JH, Lin W, Johns J, Elam M, Gerig G. Prenatal cocaine effects on brain structure in early infancy. Neuroimage. 2014;101:114–123. doi: 10.1016/j.neuroimage.2014.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Romano AG, Gabriel M, Simansky KJ, Du W, Aloyo VJ, Friedman E. Effects of prenatal exposure to cocaine on the developing brain: Anatomical, chemical, physiological and behavioral consequences. Neurotox Res. 3:117–143. doi: 10.1007/BF03033234. [DOI] [PubMed] [Google Scholar]
- Hommer RE, Seo D, Lacadie CM, Chaplin TM, Mayes LC, Sinha R, Potenza MN. Neural correlates of stress and favorite-food cue exposure in adolescents: A functional magnetic resonance imaging study. Hum Brain Mapp. 2013;34:2561–2573. doi: 10.1002/hbm.22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE. Prenatal and Adolescent Exposure to Tobacco Smoke Modulates the Development of White Matter Microstructure. The Journal of Neuroscience. 2007;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural Correlates of Stress- and Food Cue–Induced Food Craving in Obesity: Association with insulin levels. Diabetes Care. 2013;36:394–402. doi: 10.2337/dc12-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A, Scheinost D, Okuda H, Belhachemi D, Murphy I, Staib LH, Papademetris X. Unified framework for development, deployment and robust testing of neuroimaging algorithms. Neuroinformatics. 2011;9:69–84. doi: 10.1007/s12021-010-9092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse LL, Gaskins RB, Bada HS, Shankaran S, Liu J, Lester BM, Bauer CR, Higgins RD, Das A, Roberts M. Prenatal cocaine exposure and childhood obesity at nine years. Neurotoxicol Teratol. 2011;33:188–197. doi: 10.1016/j.ntt.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Coles CD, Lynch ME, Luo Y, Hu X. Longitudinal changes of amygdala and default mode activation in adolescents prenatally exposed to cocaine. Neurotoxicol Teratol. 2016;53:24–32. doi: 10.1016/j.ntt.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Ellen Lynch M, Hamann S, Peltier S, Hu X. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry Research: Neuroimaging. 2013;213:47–55. doi: 10.1016/j.pscychresns.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Santhanam P, Coles CD, Lynch ME, Hamann S, Peltier S, Hu X. Increased “default mode” activity in adolescents prenatally exposed to cocaine. Hum Brain Mapp. 2011;32:759–770. doi: 10.1002/hbm.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Interactions between the Salience and Default-Mode Networks Are Disrupted in Cocaine Addiction. The Journal of Neuroscience. 2015;35:8081–8090. doi: 10.1523/JNEUROSCI.3188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lester BM. Reconceptualizing in a dual-system model the effects of prenatal cocaine exposure on adolescent development: a short review. International Journal of Developmental Neuroscience. 2011;29:803–809. doi: 10.1016/j.ijdevneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal Brain Default-Mode Network Functional Connectivity in Drug Addicts. PLoS One. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Grillon C, Granger R, Schottenfeld R. Regulation of Arousal and Attention in Preschool Children Exposed to Cocaine Prenatally. Ann N Y Acad Sci. 1998;846:126–143. doi: 10.1111/j.1749-6632.1998.tb09731.x. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Molfese DL, Key APF, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicol Teratol. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG. Prenatal Cocaine Exposure Decreases Parvalbumin-Immunoreactive Neurons and GABA-to-Projection Neuron Ratio in the Medial Prefrontal Cortex. Dev Neurosci. 2012;34:174–183. doi: 10.1159/000337172. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Kabir ZD, Bhide PG, Kosofsky BE. Chapter 12 - Effects of prenatal exposure to cocaine on brain structure and function. In: Marco Diana GDC, Pierfranco S, editors. Prog Brain Res Elsevier. 2014. pp. 277–289. [DOI] [PubMed] [Google Scholar]
- Miller GA, Levin DN, Kozak MJ, Cook EW, McLean A, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cogn Emot. 1987;1:367–390. [Google Scholar]
- Min MO, Minnes S, Yoon S, Short EJ, Singer LT. Self-Reported Adolescent Behavioral Adjustment: Effects of Prenatal Cocaine Exposure. J Adolesc Health. 2014;55:167–174. doi: 10.1016/j.jadohealth.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Grunau R, Mayes L, Riggs W, Rurak D, Papsdorf M, Misri S, Weinberg J. Hypothalamic–pituitary–adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008;84:689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Ströhle A, Struve M, Loth E, Schumann G, Büchel C, Consortium I Lower Ventral Striatal Activation During Reward Anticipation in Adolescent Smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Potenza M, H Ki, L C, F R, T K, S R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. The American Journal of Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando K, Chaplin TM, Potenza MN, Mayes L, Sinha R. Prenatal Cocaine Exposure and Gray Matter Volume in Adolescent Boys and Girls: Relationship to Substance Use Initiation. Dev Impact Cocaine. 2013;74:482–489. doi: 10.1016/j.biopsych.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Larkby C, Goldschmidt L, Day NL. Adolescent Initiation of Drug Use: Effects of Prenatal Cocaine Exposure. J Am Acad Child Adolesc Psychiatry. 2013;52:37–46. doi: 10.1016/j.jaac.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Benjamin J, Lacadie CM, Vohr B, Schneider KC, Ment LR, Papademetris X, Constable RT. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. Neuroimage. 2012;62:1510–1519. doi: 10.1016/j.neuroimage.2012.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT. Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 2015;36:1524–1535. doi: 10.1002/hbm.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R. Sex Differences in Neural Responses to Stress and Alcohol Context Cues. Hum Brain Mapp. 2011;32:1998–2013. doi: 10.1002/hbm.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC. Cocaine addiction in mothers. Ann N Y Acad Sci. 2010a;1187:172–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC. Cocaine Addiction in Mothers: Potential Effects on Maternal Care and Infant Development. Ann N Y Acad Sci. 2010b;1187:172–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of child psychology and psychiatry, and allied disciplines. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Nitulescu I, Lewis JS, Lemos JC, Bamford IJ, Posielski NM, Storey GP, Phillips PEM, Bamford NS. Overinhibition of corticostriatal activity following prenatal cocaine exposure. Ann Neurol. 2013;73:355–369. doi: 10.1002/ana.23805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. Implications of cortical balanced excitation and inhibition, functional heterogeneity, and sparseness of neuronal activity in fMRI. Neurosci Biobehav Rev. 2015;57:264–270. doi: 10.1016/j.neubiorev.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Potenza EB, Balodis IM, Lacadie CM, Sinha R, Mayes LC, Potenza MN. Prenatal Cocaine Exposure and Adolescent Neural Responses to Appetitive and Stressful Stimuli. Neuropsychopharmacology. 2014;39:2824–2834. doi: 10.1038/npp.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin. 2017;13:181–187. doi: 10.1016/j.nicl.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


