Fig. 5.
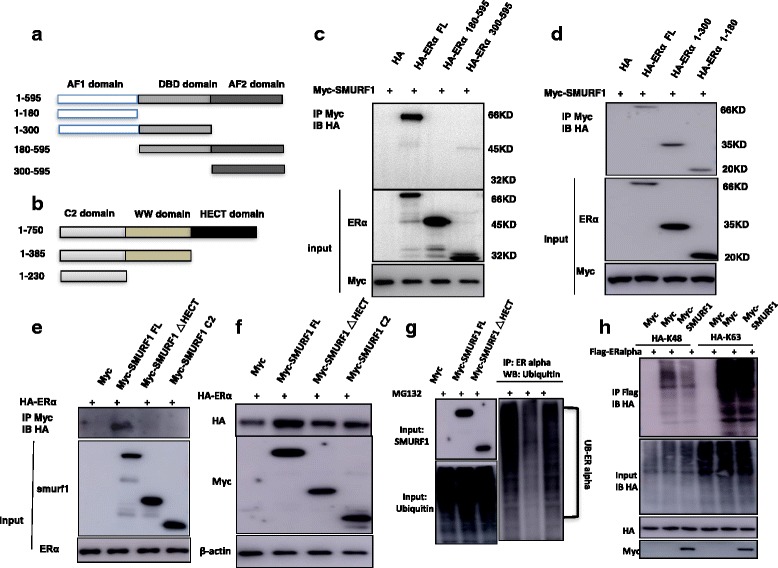
SMURF1 interacts with ER alpha AF1 domain throμgh its HECT domain and prohibits ER alpha K48 specific poly-ubiquitination. a ER alpha domain structure and deletion mutants used in the study (Full length, ΔAF1, ΔAF1 + ΔDBD, ΔAF2, ΔAF2 + ΔDBD). b SMURF1 domain structure and deletion mutants used in the study (Full length, ΔHECT, ΔHECT + ΔWW). c and d SMURF1 interacts with ER alpha throμgh its AF1 domain. HEK293 cells were transfected with 2 μg Myc-SMURF1 together with HA-ER alpha full length or mutants (ΔAF1, ΔAF1 + ΔDBD, ΔAF2 and ΔAF2 + ΔDBD). After 24 h, cells were harvested with NP-40 lysis buffer. CO-IP was performed using Myc antibody. The possible interacted ER alpha domains were detected by HA antibody. e HECT domain is required for SMURF1 interaction with ER alpha. HEK293 cells were transfected with 2 μg HA-ER alpha together with Myc-SMURF1 full length or mutants (ΔHECT, ΔHECT + ΔWW). After 24 h, cells were harvested with NP-40 lysis buffer. CO-IP was performed using HA antibody. The possible interacted SMURF domains were detected by Myc antibody. f The HECT domain is necessary for the SMURF1-mediated increase of ER alpha protein level. HEK293 cells were transfected with 2 μg HA-ER alpha together with Myc-SMURF1 full length or mutants (ΔHECT, ΔHECT + ΔWW). After 48 h, whole cell extracts were prepared and the level of ER alpha protein was assayed by western blot analysis. g The HECT domain is necessary for SMURF1 inhibition effect on ER alpha poly-ubiquitination. HEK293 cells were transfected with 2 μg Flag-ER alpha plasmid, 0.5 μg HA-Ub plasmid and 0.5 μg Myc-SMURF1/Myc-SMURF1_delta HECT/Myc-vector plasmids. The cell extracts were immunoprecipitated with ER alpha antibody. The poly-ubiquitinated ER alpha was detected by HA antibody. h SMURF1 decreases K48-linked poly-ubiquitination of ER alpha. HEK293 cells were transfected with 2 μg Flag-ER alpha plasmid, 0.5 μg HA-K48 Ubi/HA-K63 Ubi plasmid and 0.5 μg Myc-SMURF1 plasmids. The cell extracts were immunoprecipitated with HA antibody. The K48 specific poly-ubiquitinated ER alpha or K63 specific poly-ubiquitinated was detected via western blotting analysis
