Scheme 1.
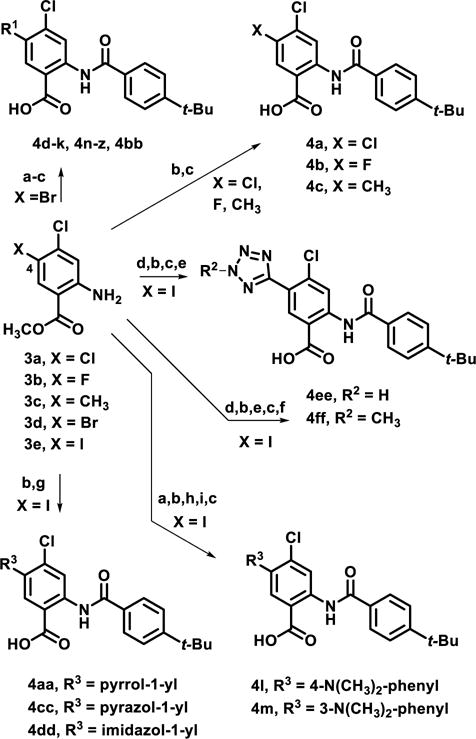
Synthesis of second generation, C4-derivatized analogs
Reagents and conditions: (a) coupling conditions, 21-92%; (b) 4-tert-butylbenzoyl chloride, CH3CN, 150°C, MW, 1 h, 25-95%; (c) LiOH, THF:H2O (1.5:1), rt, 18 h, 15-93%; (d) CuCN, DMF, 120 °C, 6 h, 78%; (e) TMSN3, CuI, DMF, MeOH, 18 h, 90 °C, 36%; (f) TMS-diazomethane, CH2Cl2:MeOH (1:1), rt, 2 h, 56-74%; (g) Cu powder, pyrrole or pyrazole, or imidazole, Cs2CO3, 150 °C, CH3CN, 27-58%; (h) Raney Ni, NaBH4, 0°C to rt, 17 h, 91%; (i) paraformaldehyde, NaCNBH3, rt, 18 h, 53%.
