Table 1.
SAR Summary of TbHK1, LmHK1 and whole parasite T. brucei BSF data for C4 analogs of 2
| entry | cmpd |
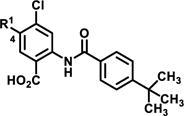
|
rTbHK1 potency (IC50 ± SEM, μM)[a] | rLmHK1 potency (IC50 ± SEM, μM)[a] | % T. brucei BSF growth inhibition (10 μM)[a] | T. brucei BSF potency (LD50 ± SEM, μM)[a] | % hGlk inhibition (10 μM)[a] | IMR90 toxicity (EC50, μM)[a] | cLogP[b] |
|---|---|---|---|---|---|---|---|---|---|
| R1 | |||||||||
| 1 | 2 | Br | 0.98 ± 0.07 | 1.10 ± 0.07 | 6.9 ± 3.0 | ND | 11.9 ± 7.2 | > 25.0 | 6.7 |
| 2 | 4a | Cl | 1.36 ± 0.06 | ND | 35.0 ± 11.0 | ND | BLD | > 25.0 | 6.6 |
| 3 | 4b | F | 2.79 ± 0.10 | ND | 32.0 ± 11.0 | ND | BLD | > 25.0 | 6.5 |
| 4 | 4c | CH3 | 2.70 ± 0.18 | ND | 97.5 ± 0.6 | 5.2 ± 1.8 | 12.5 ± 4.8 | > 25.0 | 6.5 |
| 5 | 4d | CH2CH3 | 7.40 ± 0.15 | ND | BLD | ND | 0.7 ± 1.4 | > 25.0 | 7.0 |
| 6 | 4e | cyclopropyl | 6.70 ± 0.38 | ND | 43.4 ± 16.8 | ND | BLD | > 25.0 | 6.9 |
| 7 | 4f | phenyl | 0.28 ± 0.002 | 1.70 ± 0.11 | 95.4 ± 0.8 | 1.9 ± 0.7 | 35.2 ± 8.3 | > 25.0 | 7.6 |
| 8 | 4g | 4-CH3-phenyl | 2.78 ± 0.03 | ND | 81.4 ± 5.6 | 3.2 ± 1.6 | 15.7 ± 3.0 | > 25.0 | 8.1 |
| 9 | 4h | 2-CH3-phenyl | ND | ND | 65.2 ± 0.2 | ND | 16.6 ± 2.3 | > 25.0 | 8.1 |
| 10 | 4i | 4-CH3O-phenyl | 1.72 ± 0.09 | ND | 82.5 ± 7.1 | 3.1 ± 0.2 | 22.2 ± 1.6 | > 25.0 | 7.5 |
| 11 | 4j | 3- CH3O-phenyl | 0.88 ± 0.01 | ND | 92.2 ± 2.9 | 1.5 ± 0.3 | 13.8 ± 1.9 | > 25.0 | 7.5 |
| 12 | 4k | 2- CH3O-phenyl | 4.60 ± 0.32 | ND | 90.7 ± 0.9 | 2.0 ± 0.2 | 18.8 ± 2.4 | > 25.0 | 7.5 |
| 13 | 4l | 4-N(CH3)2-phenyl | > 10.0 | ND | 58.0 ± 4.0 | 6.0 ± 0.2 | 27.8 ± 1.7 | > 25.0 | 7.8 |
| 14 | 4m | 3-N(CH3)2-phenyl | 4.00 ± 0.32 | 0.60 ± 0.09 | 82.8 ± 0.2 | 1.2 ± 0.1 | 43.7 ± 4.1 | > 25.0 | 7.8 |
| 15 | 4n | 4-F-phenyl | 2.44 ± 0.08 | ND | 76.7 ± 10.4 | 3.1 ± 0.7 | 15.1 ± 2.6 | > 25.0 | 7.8 |
| 16 | 4o | 3-F-phenyl | 4.60 ± 0.32 | ND | 93.7 ± 2.6 | 1.8 ± 0.3 | 16.1 ± 8.7 | > 25.0 | 7.8 |
| 17 | 4p | 2-F-phenyl | 2.08 ± 0.07 | ND | 92.8 ± 2.0 | 5.2 ± 0.9 | 24.5 ± 2.5 | > 25.0 | 7.8 |
| 18 | 4q | 3-Cl-phenyl | 2.70 ± 0.05 | 1.70 ± 0.22 | 90.7 ± 1.8 | 1.1 ± 0.2 | 30.9 ± 3.5 | > 25.0 | 8.3 |
| 19 | 4r | 4-CO2H-phenyl | 3.50 ± 0.33 | ND | 51.0 ± 14.3 | ND | 3.5 ± 7.1 | > 25.0 | 7.4 |
| 20 | 4s | pyridin-4-yl | > 10[c] | ND | 99.7 ± 1.4 | 3.5 ± 0.6 | 15.1 ± 3.2 | > 25.0 | 6.2 |
| 21 | 4t | pyridin-3-yl | ND[c] | ND | 57.7 ± 14.2 | ND | 16.7 ± 1.2 | > 25.0 | 6.2 |
| 22 | 4u | thiophene-2-yl | 0.47 ± 0.15 | 1.91 ± 0.04 | 69.1 ± 5.8 | ND | 12.5 ± 1.3 | > 25.0 | 7.5 |
| 23 | 4v | thiophen-3-yl | 0.33 ± 0.03 | 1.71 ± 0.03 | 68.5 ± 2.1 | ND | 24.8 ± 1.0 | > 25.0 | 7.5 |
| 24 | 4w | furan-2-yl | 0.42 ± 0.02 | 1.64 ± 0.22 | 59.0 ± 8.2 | > 10.0 | 10.4 ± 2.1 | > 25.0 | 7.0 |
| 25 | 4x | furan-3-yl | 0.51 ± 0.06 | ND | 73.7 ± 3.7 | 4.2 ± 0.4 | 3.1 ± 2.8 | > 25.0 | 7.0 |
| 26 | 4y | dihydrobenzo[1,4]dioxin-6-yl | 0.60 ± 0.09 | 0.61 ± 0.09 | 98.0 ± 1.2 | 3.3 ± 0.5 | 26.6 ± 0.4 | > 25.0 | 7.5 |
| 27 | 4z | benzo[1,3]dioxol-5-yl | 3.9 ± 0.1 | ND | 89.8 ± 1.1 | 3.0 ± 0.3 | 23.9 ± 2.5 | > 25.0 | 7.6 |
| 28 | 4aa | 1H-pyrrol-1-yl | 3.0 ± 0.2 | ND | 73.6 ± 1.0 | 6.9 ± 2.1 | 15.8 ± 3.0 | > 25.0 | 7.2 |
| 29 | 4bb | 1H-pyrrol-2-yl | 0.50 ± 0.05 | 2.06 ± 0.04 | 57.0 ± 7.1 | 6.6 ± 1.6 | 6.1 ± 0.1 | > 25.0 | 7.3 |
| 30 | 4cc | 1H-pyrazol-1-yl | 2.8 ± 0.2 | ND | 22.5 ± 12.3 | > 10.0 | 8.7 ± 4.4 | > 25.0 | 6.2 |
| 31 | 4dd | 1H-imidazol-1-yl | > 10.0 | ND | 16.4 ± 13.3 | ND | 1.7 ± 3.2 | > 25.0 | 5.9 |
| 32 | 4ee | 1H-tetrazol-5-yl | 0.14 ± 0.002 | 0.94 ± 0.16 | BLD | > 10.0 | 1.6 ± 0.5 | > 25.0 | 5.2 |
| 33 | 4ff | 2-CH3-2H-tetrazol-5-yl | > 10 | ND | BLD | ND | 14.0 ± 4.1 | > 25.0 | 5.5 |
Data were an average of n ≥ 3 experiments.
Data were calculated using CambridgeSoft ChemBioDraw Ultra 12.0.
Compound showed poor solubility which obscured analysis in this assay. ND = not determined. BLD = below level of detection.
