Abstract
Background
The aim of the study was to assess the relationship between computed tomography (CT) densitometry and routine clinical markers in patients with chronic obstructive pulmonary disease (COPD) and alpha-1 anti-trypsin deficiency (AATD).
Methods
Multiple databases were searched using a combination of pertinent terms and those articles relating quantitatively measured CT densitometry to clinical outcomes. Studies that used visual scoring only were excluded, as were those measured in expiration only. A thorough review of abstracts and full manuscripts was conducted by 2 reviewers; data extraction and assessment of bias was conducted by 1 reviewer and the 4 reviewers independently assessed for quality. Pooled correlation coefficients were calculated, and heterogeneity was explored.
Results
A total of 112 studies were identified, 82 being suitable for meta-analysis. The most commonly used density threshold was −950 HU, and a significant association between CT density and all included clinical parameters was demonstrated. There was marked heterogeneity between studies secondary to large variety of disease severity within commonly included cohorts and differences in CT acquisition parameters.
Conclusion
CT density shows a good relationship to clinically relevant parameters; however, study heterogeneity and lack of longitudinal data mean that it is difficult to compare studies or derive a minimal clinically important difference. We recommend that international consensus is reached to standardize CT conduct and analysis in future COPD and AATD studies.
Keywords: computed tomography, CT, densitometry, emphysema, chronic obstructive pulmonary disease, alpha-1 anti-trypsin deficiency
Plain language summary
Computed tomography (CT) produces a digital image that is reconstructed into the recognizable picture format. CT densitometry describes the method that uses using this information to accurately quantify the severity of emphysema, and this has been validated pathologically and clinically. However, CT densitometry is yet to be standardized and its clinical utility remains unclear. This systematic review has highlighted the vast heterogeneity that exists between studies using CT density, and despite the strong relationship to clinically relevant parameters, international consensus is still required to standardize CT conduct.
Introduction
The heterogeneity of chronic obstructive pulmonary disease (COPD) and alpha-1 anti-trypsin deficiency (AATD) is well recognized, as is the need for more descriptive biomarkers beyond lung function.1 Computed tomography (CT) has been used for many years to visually diagnose emphysema, providing the most direct assessment of its presence and distribution.2 Software programs have since been developed, which can objectively measure the severity of emphysema.3 Quantitative CT, and in particular CT densitometry, is the method of quantifying emphysema using such software; its ability to assess emphysema has been validated clinically and pathologically.4–6 However, CT densitometry is yet to be standardized, with numerous factors impacting on the measurement of density and affecting results.7 Changes seen on CT predate those seen on spirometry, with pathological studies demonstrating that up to one-third of the lung tissues is destroyed in emphysema before spirometry becomes abnormal. This suggests that CT densitometry may be a very important technique for detection of early disease, an area which is of increasing clinical interest.8 CT densitometry was the primary outcome measure for registration level randomized clinical trials (RCTs) of augmentation therapy in AATD, where signals have been seen for this measure, and only trends in the same direction for other clinical outcomes.9 More recently large cross-sectional studies in COPD have been established (eg, COPDGene), which have collected data from quantitative measures on CT as well as extensive physiology.10
Understanding the implications of density data is complex for both clinicians and regulatory agencies and no systematic reviews of its utility have been undertaken. The purpose of our study was to assess the validity of CT densitometry as a measure of severity and progression of lung disease in emphysema specifically seeking relationship to lung function, mortality, hospital admissions and quality of life (QOL).
Methods
This review is registered with Prospero (CRD42015024183). All papers concerning patients with clinically or spirometrically defined COPD that compared CT densitometry data with FEV1, gas transfer (diffusing capacity of the lungs for carbon monoxide [DLCO] or transfer factor divided by the alveolar volume [KCO]) and QOL, in the same study population were included. In addition, any study that described longitudinal density change, irrespective of whether there was a direct relationship to one of our pre-specified outcomes, was included. Studies in which COPD was secondary to AATD were included.
Population
The following databases were searched with no date or language restrictions: MEDLINE (Ovid), MEDLINE In Process (Ovid), EMBASE (Ovid), Cochrane Library (Wiley) Cochrane Central Register of Controlled Trials (CENTRAL), CMR, CDSR, HTA, NHS EED and DARE. In addition, Conference Proceedings Citation Index via Web of Science and British Library’s ZETOC was searched for conference proceedings and abstracts, and ClinicalTrials.gov and WHO International Clinical Trials Registry Platform were searched for ongoing trials. Search terms for COPD and AATD were combined with all search terms for CT or CT densitometry, and lung function (see Supplementary materials for full search terms).
Study selection
Titles and abstracts of search yield were screened for relevance by 2 reviewers independently. Disagreements were resolved by discussion, where required involving a third reviewer. Relevant articles were obtained and assessed against the full selection criteria in a similar manner (see Supplementary materials for full inclusion and exclusion criteria). Studies that used visual scoring only were excluded, as were those measured in expiration only.
Data extraction
Data were extracted using the Cochrane model, and included general study information, specifics of CT acquisition (ie, reconstruction algorithm, software and slice thickness), percentage low attenuation area (%LAA), whether the scan was taken in full inspiration, use of bronchodilator during spirometry and a CT phantom for quality assurance.11 This process was performed by 1 reviewer (DC) and checked by the remaining authors.
Risk of bias
Risk of bias was assessed by one reviewer (DC) and independently by AMT, MR, MK, and EL using a mixture of two recognized bias tools (Table S1). The AHRQ was used in order to accurately examine the large amount of cross sectional studies included, and QUADAS 2 where CT density is being considered as a diagnostic tool.12,13 Publication bias was assessed using funnel plots and Begg-Mazumdar/Egger tests, and efforts were made to reduce publication bias by using no date or language limits.
Data synthesis
Baseline characteristics are presented as mean (standard deviation) or median (interquartile range). Studies where density was taken from a single slice, where there was division into arbitrary emphysematous thresholds or that quoted mean lung density only were excluded. Studies that compared CT density with one of our chosen clinical parameters using Pearson’s correlation coefficient were meta-analyzed to estimate the Schmidt-Hunter (SH) weighted mean correlation coefficient. As SH is a random effects model, it is suitable for heterogeneous populations, with weighted means to accurately account for the variance.14 I2 and chi-square analyses were performed to assess study heterogeneity. All analyses were performed using StatsDirect, and where meta-analysis was not possible, a narrative synthesis is provided.
Results
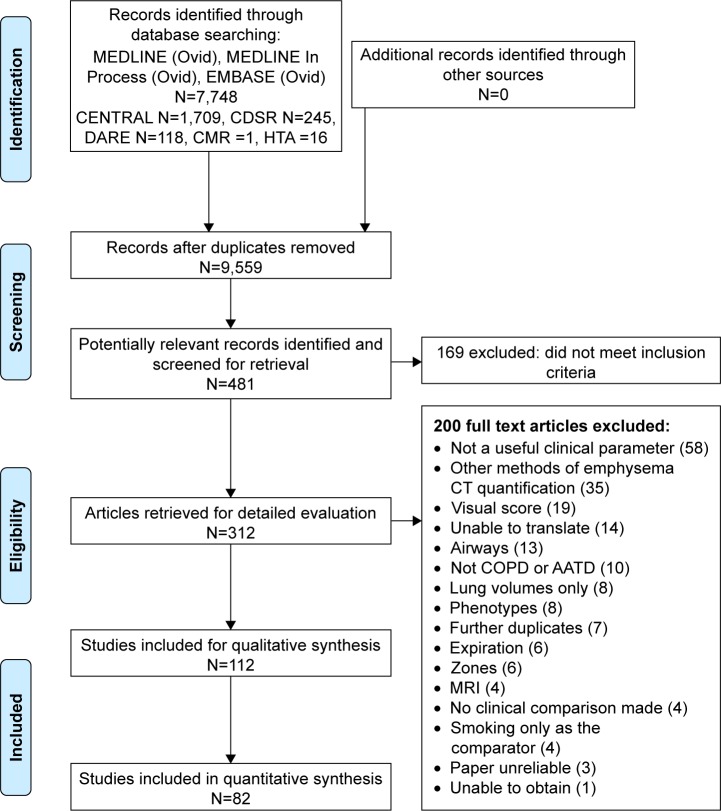
The PRISMA flow diagram (Figure 1) demonstrates that 112 papers were included in the overall narrative, and 82 papers could be combined in a quantitative meta-analysis. A small number of papers reported lobar densities, which were analyzed separately (Table S2). Characteristics of all included papers can be found in Table S3.
Figure 1.
PRISMA flow diagram.
Abbreviations: CENTRAL, Cochrane Central Register of Controlled Trials; CT, computed tomography; COPD, chronic obstructive pulmonary disease; AATD, alpha-1 anti-trypsin deficiency.
Cross-sectional studies of CT density
Pulmonary function tests
Table 1 summarizes the baseline characteristics of studies and patients included in meta-analyses. %LAA at −950 HU was the most commonly used emphysematous threshold, with 55 individual studies reporting the association between −950 and clinical parameters, of which 23 were from larger cohort studies (eg, COPDGene, KOLD).10,14
Table 1.
Summary of all studies included in meta-analyses
| Statistical method | Density measure | No of studies | No of patients | Age | FEV1pp | DLCOpp | KCOpp |
|---|---|---|---|---|---|---|---|
| Correlation coefficient | −900 | 7 | 551 | 66 (10) | 54.96 (20.19) | 65.47 (20.97) | 63.73 (20.13) |
| −910 | 2 | 69 | 64.67 (8.34) | 58.93 (24.27) | 57.46 (19.63) | 61.59 (22.47) | |
| −950 | 46 | 10,764 | 62.45 (10.77) | 58.04 (33.59) | 59.90 (31.43) | 85.19 (21.86) | |
| −960 | 6 | 639 | 67.96 (8.76) | 54.86 (23.75) | – | – | |
| PD15 | 7 | 4,544 | 60.91 (9.22) | 53.99 (24.52) | – | 67.16 (24.58) | |
| Multivariate regression | −910 | 3 | 425 | 62.04 (8.93) | 64.17 (27.27) | – | – |
| −950 | 14 | 18,984 | 60.59 (9.56) | 78.71 (26.29) | 66.23 (23.44) | 87.06 (18.07) | |
| −960 | 2 | 161 | 70.33 (8.69) | 54.81 (20.13) | – | – | |
| PD15 | 8 | 7,251 | 59.89 (9.51) | 93.06 (20.63) | – | 85.19 (18.86) | |
| Trials | |||||||
| • ICS ± LABA | −950 | 2 | 482 | 64.50 (7.37) | 50.50 (12.21) | 75.15 (29.50) | 46.70 (39.08) |
| • ATRA | PD15 | 2 | 375 | 58.83 (9.91) | 44.99 (15.70) | 43.82 (14.79) | 43.70 (13.57) |
| • Prolastin | PD15 | 4 | 369 | 51.83 (7.39) | 47.39 (12.35) | 36.14 (23.39) | 54.70 (11.77) |
| Mortality | Mixed | 6 | 3,584 | 61.66 (9.68) | 69.39 (31.27) | – | – |
| Exacerbations | Mixed | 7 | 2,637 | 66.10 (8.22) | 60.54 (25.44) | – | – |
| SGRQ | Mixed | 8 | 4,864 | 58.82 (13.75) | 45.01 (19.03) | 35.68 (18.17) | 60.40 (22.22) |
| BODE | Mixed | 4 | 2,440 | 65.58 (6.44) | 44.43 (21.19) | 35.08 (19.87) | – |
| 6MWT | Mixed | 3 | 2,481 | 61.63 (9.38) | 56.03 (48.57) | – | – |
| MRC | Mixed | 4 | 694 | 64.84 (10.85) | 56.97 (18.99) | – | – |
Notes: Studies of lung function, sub-divided by statistical techniques, were used to assess relationship to CT density, followed by trials, and those using quality of life measures. All quantitative measures are shown as mean (SD). ‘–’ indicates data not available.
Abbreviations: FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of the lungs for carbon monoxide; KCO, transfer factor divided by the alveolar volume; pp, percent predicted; PD15, 15th percentile point; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; ATRA, all-trans retinoic acid; SGRQ, St Georges Respiratory Questionnaire; BODE, BMI, airflow obstruction, dyspnea and exercise tolerance; 6MWT, 6-minute walk test; MRC, Medical Research Council; CT, computed tomography.
Spirometry
FEV1
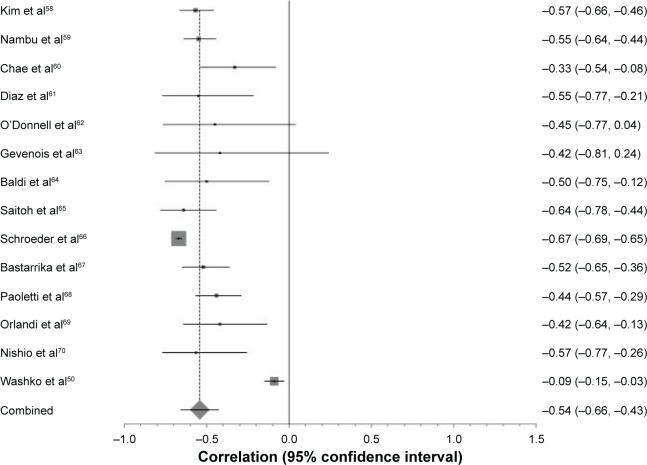
A total of 36 studies compared forced expiratory volume in 1 second (FEV1) percent predicted to CT density. The forest plot in Figure 2 demonstrates the correlation between FEV1 percent predicted with CT density at −950 HU, and the variation between the included studies. These data are summarized in Table 2, which shows meta-analyses of the other CT parameters against FEV1 (L) or FEV1 percent predicted. The level of heterogeneity remained high in all sub-group analyses except for 900 HU and FEV1 percent predicted, which contained the smallest number of studies and thus could be less reliable.
Figure 2.
Forest plot of all studies included in the meta-analysis that correlated FEV1 percent predicted with −950 HU.
Notes: The ranges of correlation coefficients are from −0.09 to −0.67. Pooled correlation coefficient =−0.54 (p<0.0001), χ2 test for heterogeneity =591 and I2 score for inconsistency =97.2%.
Abbreviation: FEV1, forced expiratory volume in 1 second.
Table 2.
Summary of meta-analyses performed on all studies using Pearson’s correlation coefficient to compare FEV1 and FEV1 percent predicted with CT density
| PFT | Density variable | No of studies | SH pooled correlation | Lower 95% CI | Higher 95% CI | p-value | I2 | χ2 | p-value |
|---|---|---|---|---|---|---|---|---|---|
| FEV1 | −950 | 6 | −0.37 | −0.53 | −0.21 | <0.0001 | 90.7 | 48.54 | <0.0001 |
| −960 | 3 | −0.33 | −0.43 | −0.22 | <0.0001 | 46.9 | 3.78 | 0.15 | |
| PD15 | 4 | 0.40 | 0.20 | 0.59 | <0.0001 | 84.9 | 18.90 | 0.0003 | |
| FEV1pp | −900 | 3 | −0.53 | −0.63 | −0.43 | <0.0001 | 0 | 1.96 | 0.37 |
| −910 | 9 | −0.29 | −0.38 | −0.20 | <0.0001 | 80.5 | 36.45 | <0.0001 | |
| −950 | 14 | −0.54 | −0.66 | −0.42 | <0.0001 | 97.2 | 591.46 | <0.0001 | |
| −960 | 4 | −0.35 | −0.51 | −0.19 | 0.0003 | 84.5 | 17.70 | 0.0005 | |
| PD15 | 4 | 0.45 | 0.27 | 0.63 | <0.0001 | 98.8 | 288.78 | <0.0001 |
Notes: SH weighted mean correlation coefficient, heterogeneity scores (I2) and chi-square values (χ2) are shown.
Abbreviations: FEV1, forced expiratory volume in 1 second; CT, computed tomography; SH, Schmidt-Hunter; CI, confidence interval; PD15, 15th percentile point; FEV1pp, forced expiratory volume in one second percent predicted; PFT, pulmonary function test.
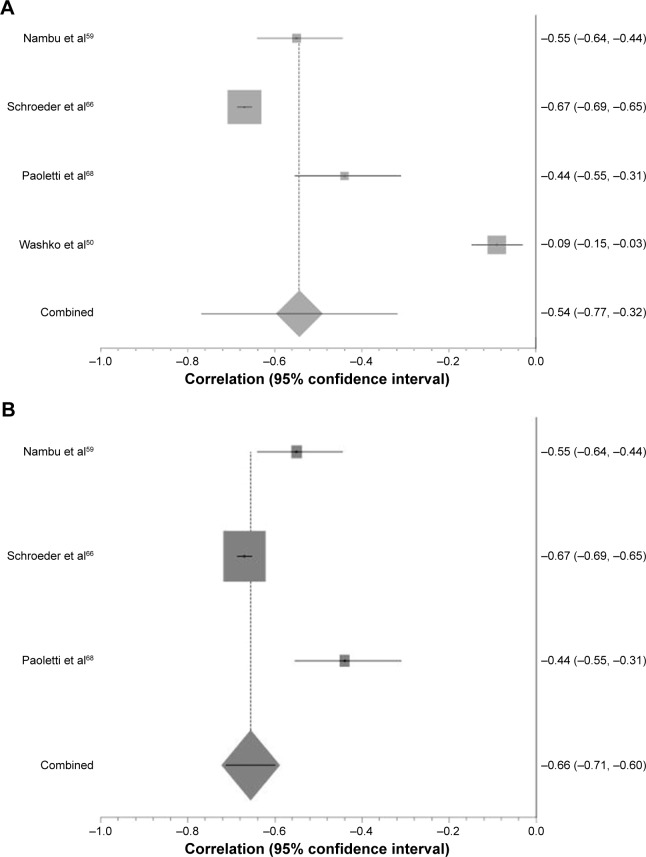
Further investigation into the cause of heterogeneity revealed major differences in the choice and combination of reconstruction algorithm, slice thickness and software program used by included studies. When meta-analysis was restricted to those studies using the same CT acquisition parameters, the forest plot became more uniform and heterogeneity reduced (Figure 3).
Figure 3.
The effect of CT algorithm on heterogeneity of results with respect to −950 HU and FEV1 percent predicted.
Notes: (A) Forest plot demonstrating individual Pearson’s correlation coefficients and pooled result for those studies comparing −950 HU and FEV1 percent predicted. SH weighted mean correlation coefficient =−0.54, I2=99.3%, χ2=587.85. (B) Forest plot demonstrating the effect on heterogeneity once the same reconstruction algorithm, slice thickness and software program were used. SH weighted mean correlation coefficient =−0.66, I2=91.8%, χ2=33.59.
Abbreviations: CT, computed tomography; FEV1, forced expiratory volume in 1 second; SH, Schmidt-Hunter.
Seven studies performed multivariate linear regression between FEV1 and CT density (Table 3). However, for each density variable, all studies were adjusted for different variables and therefore an accurate meta-analysis could not be performed.
Table 3.
Summary of studies that performed multivariate linear regression analyses to examine the relationship between FEV1 and CT density
| Density measure | Study | Variables adjusted for | Results for adjusted FEV1 | 95 % CI |
|---|---|---|---|---|
| −950 HU | Kim et al16 | Mean wall area Visual score of emphysema Visual score of lobe no with AWT |
β=−0.4726 | −0.8215, −0.1238 |
| Mohamed Hoesein et al17 | Age Height Pack years Smoking status |
β=−0.252 | – | |
| Hong et al18 | %LAA −950 HU Mean lung density Mean wall area Smoking status |
β=−0.24 | – | |
| Aziz et al19 | DLCO FEV1 |
β=−1.27 | −1.59, −0.94 | |
| PD15 | Mohamed Hoesein et al20 | Age FEV1/FVC Medical center Mucus production Smoking status |
1 point change in PD15 results in a −0.824 mL 3-year change in FEV1 | −1.473, −0.0174 |
| Mohamed Hosein et al21 | Age Height Medical center Smoking status Years in study |
1 HU change in PD15 results in a −4.75 mL 3-year change in FEV1 | −3.3, −6.1 | |
| Mohamed Hoesein et al22 | Age FEV1 Pack years |
10 HU drop in Perc15 caused a −10 mL change in FEV1 | −15, −5 |
Note: ‘–’ indicates data not available.
Abbreviations: FEV1, forced expiratory volume in 1 second; CT, computed tomography; CI, confidence interval; AWT, airway wall thickness; %LAA, percentage low attenuation area; DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity; PD15, 15th percentile point.
FEV1/forced vital capacity
Akin to FEV1, there was a significant correlation between each density variable and FEV1/forced vital capacity (p<0.0007; Table 4). Again, large visual and statistical heterogeneity was improved by restricting to studies using the same CT parameters (Figure 4).
Table 4.
Summary of studies comparing FEV1/FVC with CT density, divided up in to the most commonly reported thresholds
| PFT | Density variable | No of studies | SH pooled correlation | Lower 95% CI | Higher 95% CI | p-value | I2 | χ2 | p-value |
|---|---|---|---|---|---|---|---|---|---|
| FEV1/FVC | −910 | 5 | −0.33 | −0.49 | −0.16 | <0.0001 | 95.5 | 75.72 | <0.0001 |
| −950 | 14 | −0.38 | −0.53 | −0.23 | <0.0001 | 95.6 | 251.72 | <0.0001 | |
| −960 | 3 | −0.48 | −0.71 | −0.25 | <0.0001 | 88.9 | 18.73 | <0.0001 | |
| PD15 | 6 | 0.26 | 0.09 | 0.43 | 0.0022 | 94.9 | 81.78 | <0.0001 |
Note: Heterogeneity score (I2), chi-square value (χ2) and SH weighted mean correlation coefficient are shown.
Abbreviations: FEV1/FVC, forced expiratory volume in 1 second/forced vital capacity; CT, computed tomography; SH, Schmidt-Hunter; CI, confidence interval; PD15, 15th percentile point.
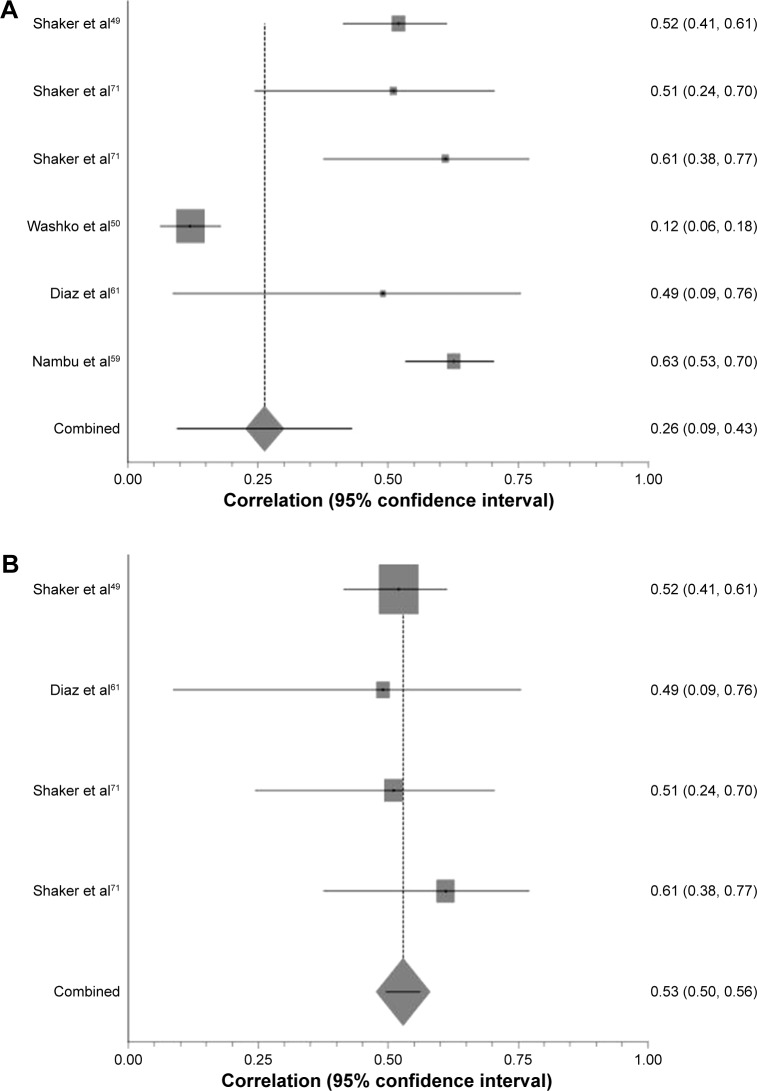
Figure 4.
The effect of CT algorithm on heterogeneity of results with respect to PD15 and FEV1/FVC.
Notes: (A) Forest plot of all studies comparing FEV1/FVC with PD15. SH weighted mean correlation coefficient =−0.26, I2=94.9%, χ2=81.78. (B) Forest plot of all studies comparing FEV/FVC with PD15 using the same CT parameters. SH weighted mean correlation coefficient =−0.47, I2=4.1%, χ2=3.23.
Abbreviations: CT, computed tomography; PD15, 15th percentile point; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SH, Schmidt-Hunter.
Gas transfer
A total of 23 studies compared DLCO percent predicted to CT density. The pooled correlation coefficients were universally significant across each of the density values, albeit slightly weaker than for FEV1 percent predicted and CT density (Table 5). The same pattern was seen regarding heterogeneity of results, with I2 dropping from 91.5% to 0 once CT algorithm was taken into account (Figure 5).
Table 5.
Studies subdivided into density parameter used, which compares gas transfer to CT measured density
| PFT | Density variable | No of studies | SH pooled correlation | Lower 95% CI | Higher 95% CI | p-value | I2 | χ2 | p-value |
|---|---|---|---|---|---|---|---|---|---|
| DLCO | −950 | 5 | −0.42 | −0.53 | −0.32 | <0.0001 | 77.8 | 15.538 | 0.0036 |
| DLCOpp | −910 | 3 | −0.31 | −0.40 | −0.22 | <0.0001 | 83.6 | 10.46 | 0.005 |
| −950 | 16 | −0.43 | −0.52 | −0.34 | <0.0001 | 88 | 100.34 | <0.0001 | |
| PD15 | 5 | 0.29 | 0.15 | 0.42 | <0.0001 | 92.2 | 33.79 | <0.0001 | |
| KCO | −950 | 3 | −0.63 | −0.71 | −0.54 | <0.0001 | 49.9 | 4.43 | 0.1091 |
| PD15 | 3 | 0.38 | 0.15 | 0.61 | 0.0012 | 96.4 | 45.67 | <0.0001 | |
| KCOpp | −910 | 3 | −0.61 | −0.63 | −0.59 | <0.0001 | 0 | 0.29 | 0.8658 |
| −950 | 6 | 0.42 | −0.6 | −0.25 | <0.0001 | 78.6 | 22.47 | 0.0004 |
Note: Heterogeneity score (I2); chi-square value (χ2) and SH weighted mean correlation coefficient are shown.
Abbreviations: CT, computed tomography; SH, Schmidt-Hunter; CI, confidence interval; DLCO, diffusing capacity of the lungs for carbon monoxide; pp, percent predicted; PD15, 15th percentile point; KCO, transfer factor divided by the alveolar volume.
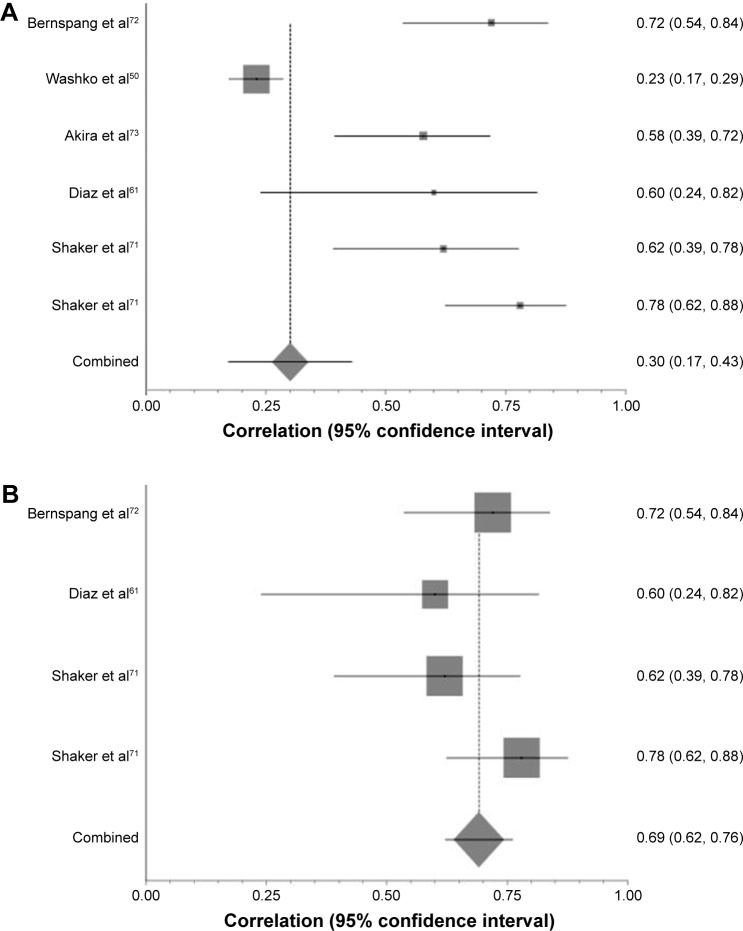
Figure 5.
The effect of CT algorithm on heterogeneity of results with respect to PD15 and DLCO percent predicted.
Notes: (A) Forest plot demonstrating correlation coefficient confidence intervals and pooled correlation coefficient for those studies comparing DLCO percent predicted with PD15. SH weighted mean correlation coefficient =0.3, I2=91.5%, χ2=40.98. (B) Forest plot of those studies comparing PD15 and DLCO percent predicted once all studies using the same CT variables have been re-analyzed. SH weighted mean correlation coefficient =0.69, I2=0%, χ2=2.72.
Abbreviations: CT, computed tomography; PD15, 15th percentile point; DLCO, diffusing capacity of the lungs for carbon monoxide; SH, Schmidt-Hunter.
Quality of life, symptom and composite scores
St Georges Respiratory Questionnaire (SGRQ) was the most frequently reported measure of QOL compared with CT density. A total of 2 out of 5 studies using correlation coefficients showed no relationship between the two measures, while the other 3 showed a strong association (p<0.003) (Table 6). There was variability in the density threshold and patient groups used (eg, cancer screening populations or those being considered for lung volume reduction surgery), thus precluding meta-analysis. Nevertheless studies that performed multivariate analyses consistently showed a significant association between density and SGRQ.
Table 6.
Summary of studies that correlated CT density with SGRQ
| References | COPD or AATD | %LAA parameter | Statistical technique | Results | p-value |
|---|---|---|---|---|---|
| Univariate analysis | |||||
| Stolk et al23 | AATD | PD15 | Spearman’s CC | −0.56 | <0.007 |
| −950 HU | 0.6 | 0.003 | |||
| Dowson et al24 | AATD | −910 | Spearman’s CC | 0.39 | <0.001 |
| Barjaktarevic et al25 | COPD | −950 | Pearson’s CC | 0.028 | 0.572 |
| Motohashi et al26 | COPD | −940 | Pearson’s CC | 0.501 | <0.001 |
| de Torres et al27 | COPD | −960 | Pearson’s CC | −0.12 | 0.39 |
| Martinez et al28 | COPD | −950 | Un-normalized and normalized (value – mean/SD) parameters in univariate analysis | Un-normalized estimate 0.53 (95% CI 0.45, 0.61) Normalized estimate 5.82 (95% CI 4.91, 6.72) |
<0.001 <0.001 |
| Multivariate analysis | |||||
| Martinez et al29 | COPD | −950 | Adjusted for age, pack years and FEV1 percent predicted | Beta value =−7.69 (95% CI −14.09, −1.3) | 0.02 |
| Gietema et al30 | COPD | −950 | Adjusted for sex, age, smoking status, pack years, BMI and FEV1 percent predicted and Pi10 | Coefficient =1.43 SE =0.57 |
<0.05 |
Abbreviations: CT, computed tomography; SGRQ, St Georges Respiratory Questionnaire; COPD, chronic obstructive pulmonary disease; AATD, alpha-1 antitrypsin deficiency; %LAA, percentage low attenuation area; PD15, 15th percentile point; CC, correlation coefficient; SD, standard deviation; CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in 1 second; Pi10, 10 mm luminal perimeter; SE, standard error.
Studies of BMI, airflow obstruction, dyspnea and exercise capacity and Medical Research Council (MRC) versus CT density used different density thresholds and statistical techniques but again showed strong relationships between density and the score in question in multivariate analyses (Table 7).
Table 7.
Summary of studies that compare BODE and MRC with CT density, subdivided into univariate or multivariate models used
| References | COPD or AATD | Severity measure | %LAA | Statistical technique | Results | p-value |
|---|---|---|---|---|---|---|
| Univariate analysis | ||||||
| Camiciottoli et al31 | COPD | BODE | −950 | Pearson’s CC | R=0.58 | <0.0001 |
| Martinez et al28 | COPD | BODE | −950 | Un-normalized and normalized (value – mean/SD) parameters in univariate analysis | Un-normalized estimate 1.02 (95% CI 1.02–1.02) Normalized estimate 1.23 (95% CI 1.2–1.26) |
<0.001 <0.001 |
| de Torres et al27 | COPD | BODE | −960 | Pearson’s CC | R=−0.08 | 0.53 |
| Camiciottoli et al6 | COPD | MRC | −950 | Odds ratio | 1.41 (95% CI 1.11–1.78) | <0.005 |
| Haruna et al32 | COPD | MRC | −960 | Pearson’s CC | R=0.41 | <0.05 |
| Haruna et al33 | COPD | MRC | −960 | Pearson’s CC | 0.41 | <0.05 |
| de Torres et al27 | COPD | MRC | −960 | Pearson’s CC | R=−0.19 | 0.14 |
| Multivariate analysis | ||||||
| Martinez et al29 | COPD | BODE | −910 | Adjusted for age, pack years and FEV1% predicted | Beta value =0.01 7.69 (95% CI 0.005, 0.02) | 0.002 |
| Camiciottoli et al31 | COPD | BODE | −950 | Adjusted for FEV1, BMI, MRC, 6MWT | R=0.61 | <0.0001 |
| Haruna et al32 | COPD | MRC | −960 | Adjusted for FEV1, RV/TLC R5-R20, X5 | R=0.06 | <0.05 |
Abbreviations: BODE, BMI, airflow obstruction, dyspnea and exercise tolerance; MRC, Medical Research Council; CT, computed tomography; COPD, chronic obstructive pulmonary disease; AATD, alpha-1 antitrypsin deficiency; %LAA, percentage low attenuation area; CC, correlation co-efficient; SD, standard deviation; CI, confidence interval; MRC, Medical Research Council; 6MWT, 6-minute walk test; FEV1, forced expiratory volume in 1 second; RV/TLC, residual volume/total lung capacity; R5-R20, measure of airway resistance at 5 and 20 Hz; X5, airway reactance at 5 Hz.
Longitudinal studies of CT density
Mortality
A total of 6 papers reported the relationship between CT density and mortality, 3 of which provided a hazard ratio for all-cause mortality (Table 8) generated by multi-variable logistic regression. However, it was inappropriate to combine them statistically due to differing emphysematous thresholds and confounding variables included in their models. Emphysema as defined by CT density remained a significant independent predictor for mortality throughout.
Table 8.
Studies reporting an all-cause mortality HR for emphysema as defined by CT density
| References | Patient source | %LAA | Statistical technique | Confounding variables in model | Results | HR | Lower CI | Upper CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Haruna et al33 | COPD OPA clinic | −960 | Univariate and multivariate Cox proportional hazards | Univariate analysis and therefore N/A | ↑%LAA significantly related to mortality | 1.52 | 1.2 | 1.91 | <0.001 |
| Upper lung field | 1.55 | 1.22 | 1.95 | <0.001 | |||||
| Lower lung field | 1.41 | 1.09 | 1.78 | 0.009 | |||||
| Age, BMI, FEV1, RV/TLC and KCO | %LAA independent predictor of mortality | 1.74 | 1.18 | 2.54 | <0.01 | ||||
| Martinez et al34 | NETT | −950 | Univariate and multivariate Cox proportional hazards | N/A | Whole lung % emphysema not associated with mortality | 1.14 | 0.85 | 1.52 | 0.38 |
| Lower zone emphysema associated with ↑mortality | 1.39 | 1.04 | 1.85 | 0.02 | |||||
| Age, LTOT, Hb, BODE, RV%, TLC%, DLCO%, maximal CPET workload, lower lung emphysema and nuclear perfusion scan result | Difference between upper and lower lungs % emphysema remained predictive in multivariate model | 1.80 | 1.22 | 2.66 | 0.003 | ||||
| Dawkinset al35 | ADAPT | −910 | Univariate Cox proportional hazards | Age HR for mortality (Exp B) comparing those with FEV1>80 pp with FEV1<30 pp |
Survival curves indicate a relationship of ↑VI to ↑mortality ↑%LAA associated with ↑mortality | 0.111 | 0.026 | 0.473 | 0.003 |
Note:↑ represents as increased.
Abbreviations: HR, hazard ratio; CT, computed tomography; %LAA, percentage low attenuation area; CI, confidence interval; COPD, chronic obstructive pulmonary disease; N/A, not applicable; BMI, body mass index; FEV1, forced expiratory volume in 1 second; RV, residual volume; TLC, total lung capacity; KCO, transfer factor divided by the alveolar volume; LTOT, long-term oxygen therapy; Hb, hemoglobin; BODE, BMI, airflow obstruction, dyspnea and exercise tolerance; DLCO, diffusing capacity of the lungs for carbon monoxide; CPET, cardiopulmonary exercise testing; pp, percent predicted; VI, voxel index; OPA, outpatient; NETT, National Emphysema Treatment Trial; ADAPT, Antitrypsin Deficiency Assessment and Programme for Treatment.
Exacerbations
A total of 4 studies investigated low CT density as a risk factor for COPD exacerbations using multiple regression analyses in order to independently attribute exacerbations to density loss (Table 9). Due to different statistical methods, and the variables adjusted for, a statistical meta-analysis could not be performed. All but 1 study showed a significant relationship between CT density and exacerbations; Yoo et al found that the ability for emphysema index to predict exacerbations did not remain significant when numerous variables such as age, SGRQ and Charlson Index score were included in multiple regression analysis.36 Cheng et al performed a multivariate ordinal logistic regression to demonstrate that %LAA >7.5 was associated with worse performance status and MRC grade if they presented to Accident and Emergency with an infective exacerbation of COPD.37
Table 9.
Summary of papers describing the association between CT density and exacerbations, subdivided into the risk of exacerbations from a low density score, and the impact exacerbations have on CT density decline
| References | %LAA parameter | Statistical technique | Variable adjusted for | Results | p-value |
|---|---|---|---|---|---|
| Yoo et al36 | −950 | Univariate logistic regression | OR =1.02 (95% CI 1.01, 1.04) | 0.01 | |
| Multiple logistic regression | Sex, gender, current smoker, exacerbation leading to hospitalization in past year, Charlson index, BMI, MMRCS, 6MWD, SGRQ, FEV1%, CT wall area %, CT air trapping index | OR =1.01 (95% CI 0.987, 1.034) | 0.39 | ||
| Vijayasaratha and Stockley38 | PD15 | Stepwise linear regression; spearman’s CC | FEV1, FEV/FVC, KCO% predicted, delay in treatment initiation in days, Anthonisen criteria, cold symptoms | PD15 associated with exacerbation length and (r=−0.361) Treatment delay (r=−0.786) |
0.003 0.004 |
| McAllister et al39 | −910 | Multivariate RR | Age, sex, race/ethnicity and cotinine | % Emphysema predicts episodes of care RR 1.45 (95% CI 1.04, 2.03) | 0.03 |
| ↑Hospital admissions RR 1.62 (95% CI 1.08, 2.44) | 0.02 | ||||
| Han et al40 | −950 | Multivariate analyses and forward selection regression | Scanner model, age, sex, smoking status and FEV1 | >35% emphysema associated with a 1.18-fold increase in exacerbation | 0.047 |
| 5% ↑in emphysema associated with a 0.86-fold ↑in exacerbation frequency | 0.001 |
Note:↑ represents as increased.
Abbreviations: CT, computed tomography; %LAA, percentage low attenuation area; OR, odds ratio; CI, confidence interval; BMI, body mass index; MMRCS, Modified Medical Research Council Dyspnea Scale; 6MWD, 6-minute walk distance; SGRQ, St Georges Respiratory Questionnaire; FEV1, forced expiratory volume in 1 second; PD15, 15th percentile point; CC, correlation co-efficient; FVC, forced vital capacity; KCO, transfer factor divided by the alveolar volume; RR, rate ratios.
Interventional studies reporting CT density
AAT augmentation therapy
A total of 3 RCTs used CT density as an outcome measure for augmentation therapy in AATD patients;9,41,42 change in CT density was the primary outcome in 2 studies41,42 and secondary outcome in the earliest work.9 A fourth paper was not included in the quantitative synthesis as it simply explored statistical approaches in data from the EXACTLE trial.43 In all papers, CT density was log transformed and volume adjusted (see Table S3 for CT acquisition parameters), study duration was 2–3 years and the rate of density decline was measured in g/l−1 per year. A recent meta-analysis of these data has been reported separately, which demonstrates slower density decline in those receiving augmentation therapy than those receiving placebo (p=0.002).44 The 3 papers analyzed also report overall low to moderate correlation coefficients between CT density and FEV1, KCO and exercise tolerance (0.31, 0.47 and −0.21 respectively).
All-trans retinoic acid
All-trans retinoic acid was shown to promote alveolar repair in animal models and subsequently 2 studies examined its effect in AATD as measured by CT density. However, neither showed any significant benefit on density decline nor did either comment on the observed relationship between CT density and other clinical parameters.45,46
Inhaled long-acting beta agonist/inhaled corticosteroid
A total of 2 studies from South Korea and the KOLD study collected longitudinal data on spirometric change over 3 months with inhaled corticosteroid/long-acting beta agonist treatment and demonstrated a significant correlation between FEV1 and baseline CT density using −950 HU as the emphysematous threshold.47,48 Shaker et al performed annual CT densitometry in a RCT conducted in patients with COPD, which demonstrated significantly slower decline in emphysema (using −910 HU; p=0.02) in those randomized to budesonide compared to placebo.49
Standardizing studies for equal CT variables
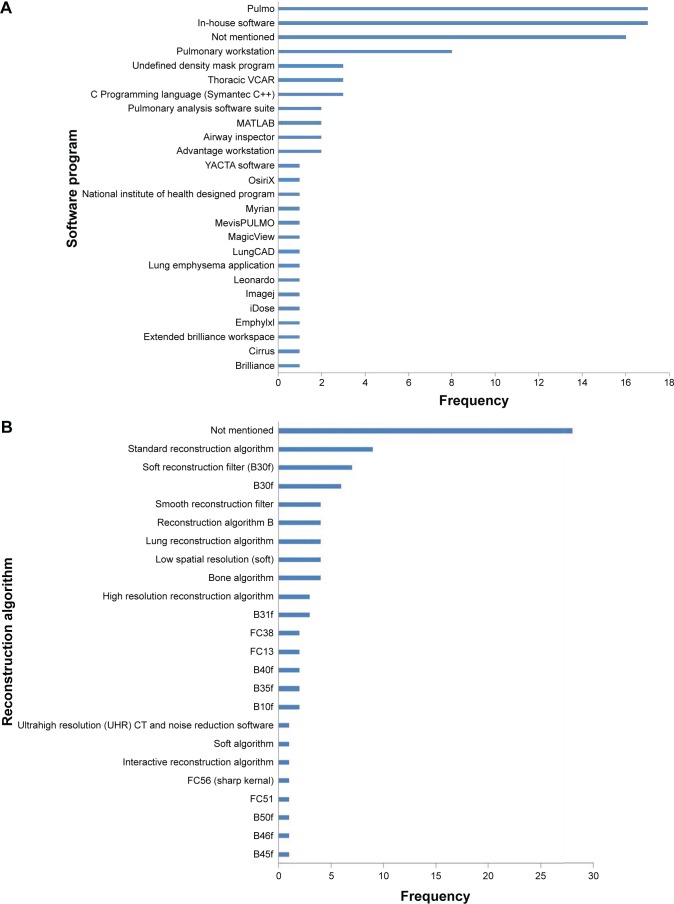
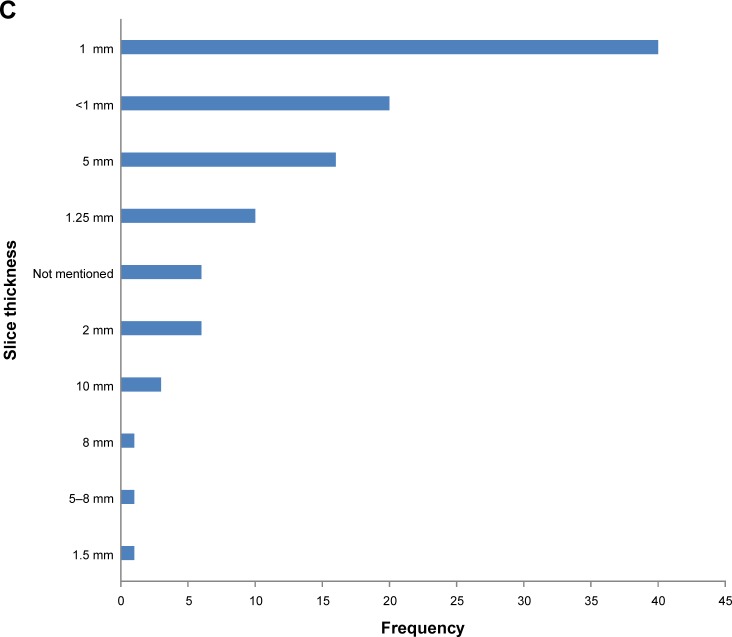
Since there were clear differences in the meta-analyses regarding the relationship between CT density and outcome when stratified by CT algorithm/statistical methods, we felt it was important to summarize the wide range of methods used in included studies (Figure 6).
Figure 6.
(A) Bar chart to demonstrate variety of software programs used in all studies. (B) Bar chart to demonstrate the variety of reconstruction algorithms reported. (C) Bar chart to demonstrate variety of slice thicknesses reported in all studies.
Pulmo was the most frequently used software program (used in EXACTLE and RAPID trials), followed by Pulmonary Workstation (used in COPDGene studies).
Most commonly, the reconstruction algorithm used was not mentioned, followed by “standard reconstruction algorithm”. The reconstruction algorithms are scanner specific, not always clear and therefore would be difficult to reproduce. The most frequently used slice thicknesses were 1 mm (N=40) and sub-millimeter (N=20).
Bias assessment
Risk of bias is summarized in Table 10 (see Table S4 for the full risk of bias assessment). Bias introduced during patient selection was relatively low, with the patient source, selection and inclusion/exclusion criteria well described. The highest level of uncertainty that could have led to bias was within index and reference test (ie, CT and lung function). There was variability in the detail that authors gave with regards to CT acquisition, the use of a phantom and whether or not a bronchodilator was applied before spirometry was performed. Missing data and confounding variables were often not accounted for, but the statistical tests applied were considered appropriate.
Table 10.
Summary of the risk of bias assessment
| Risk of bias | Low | High | Unclear |
|---|---|---|---|
| Patient selection | 81 (72) | 8 (7) | 24 (21) |
| Index and reference test | 36 (32) | 8 (7) | 69 (61) |
| Flow and timing | 60 (53) | 28 (25) | 25 (22) |
| Reporting | 59 (52) | 39 (35) | 15 (13) |
Note: Data presented as number of studies (% of studies).
Publication bias
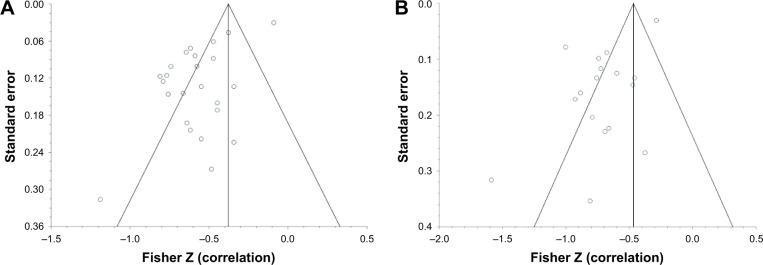
The funnel plots for −959 HU versus FEV1 percent predicted and DLCO percent predicted (analyses containing the most studies) show a significant degree of publication bias (Figure 7). On further inspection of both plots, there is one study with a low standard error and large population (Washko et al) that causes the funnel plots to shift to the right.50 Without this study, it stands to reason that the funnel would be more inclusive of the studies within the plot and could imply less publication bias. The risk of bias by two independent reviewers of this study was concluded as moderate, based on no mention of the time interval between the CT and pulmonary function tests. There was also no explanation of how confounding variables were assessed or controlled for, or how missing data were handled.
Figure 7.
(A) Funnel plot for studies correlating −950 HU with FEV1 percent predicted. (B) Funnel plot for studies correlating −950 HU with DLCO percent predicted.
Abbreviations: FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of the lungs for carbon monoxide.
Discussion
The purpose of this review was to summarize all the currently available literature regarding CT density and its association with commonly used clinical parameters to develop a clear understanding of the utility of CT density measures for current and future clinical practice. This is particularly important as CT density has been used as a primary outcome in registration level clinical trials in AATD, but doubt has been cast by some authors as to its relevance as a surrogate outcome.41,51 Our data showed that association between CT density and other clinical parameters deemed suitable as outcomes for airways disease trials (eg, FEV1, SGRQ) were consistently significant, and furthermore there was a clear and consistent relationship to mortality. This suggests that CT density is an appropriate surrogate outcome measure in studies of emphysema, like those conducted in AATD. However, publication and other biases as well as study heterogeneity make it more difficult to draw conclusions regarding the precise strength of each relationship.
Over half of the included studies were from larger cohort studies and subsequent retrospective/cross-sectional analysis. The nature of these cohorts introduces heterogeneity in the types of patients recruited, ie, lung cancer screening studies (NELSON),52 alpha one cohorts,53 COPD (eg, COPDGene, KOLD15,54) and end-stage disease (eg, NETT).55 The consistency of direction of the relationship between density and lung function across diverse patient groups is reassuring and suggests that density could be a valid surrogate outcome across the spectrum of disease severity. However, the wide range of values seen for the CT versus FEV1 correlations meant that defining the exact level of CT density that relates to, for instance, the minimal clinically important difference (MCID) for FEV1 was difficult.
The chosen CT parameter (eg, −950 HU, 15th percentile point [PD15]), software program, reconstruction algorithm and slice thickness varied grossly throughout. This level of heterogeneity was far greater than we had anticipated and made combination of all data via meta-analysis potentially less valid. The broad range of published correlation coefficients seen between −950 HU and FEV1% (Figure 2) (from −0.1 to −0.8) demonstrated this well, and when only studies with the same CT acquisition variables were analyzed, the level of heterogeneity fell dramatically. This implies that future CT density studies should have a standardized approach. Despite PD15 being established as the most reliable and sensitive measure, we have seen many studies that do not use this parameter, and would encourage authors to report this value so that data can be combined and our knowledge can grow.
The most appropriate CT algorithm would be a soft reconstruction algorithm (eg, B30f), slice thickness 2.5–5 mm and a software program that yields reliable and repeatable results. The algorithm and slice thickness are optimal due to minimal technical noise. Sharper algorithms and thinner slices have been demonstrated to overestimate the amount of emphysema.56 Many publications used in-house software that, while producing useful data, may not be comparable to one another.57 For example, Pulmo and Pulmonary Workstation are two of the most commonly used software programs (used in RAPID trial and COPDGene cohort studies, respectively), and if identical and repeatable results can be produced by both programs then cohort studies using them can then be combined and meta-analyzed to increase power. This requires direct comparison of the software on the same scans; a similar approach would be needed for slice thickness, reconstruction and so on. There are limited studies of this nature to date.7
There was a paucity of longitudinal CT density data in the included studies, which precluded conclusions about the sensitivity and specificity of CT density change over time with respect to our chosen outcomes. This means that we are unable to assess the relationship between CT density and clinical parameters over time for which there was a known MCID (eg, FEV of 100 mL), and therefore a proposal of a MCID for CT density was not possible. This would be of particular use for registration level trials, which have used or intend to use this as their primary outcome.
The key strengths of this review are that it was very broad; therefore, all potential papers were captured. Rigorous checking of data extracted from the large number of included studies was done, and the statistical analyses were conducted under supervision of an experienced statistician. Limitations were largely centered on the quality and heterogeneity of the included studies. There are other CT scanner variables that we did not examine in more detail as their impact was considered less relevant, eg, scanner type and radiation dose. There were 14 papers in languages to which we did not have access to a translator such as Japanese and Korean.
Conclusion
This evidence synthesis has demonstrated that CT density relates significantly to all commonly used clinical parameters. However, the large amount of heterogeneity and lack of longitudinal data mean that how sensitive and specific CT density is to change relating to time or interventions is not clear. We recommend that international consensus be reached to standardize CT conduct and analysis in future emphysema studies.
Acknowledgments
The authors would like to thank Peter Nightingale, the statistician responsible for reviewing the meta-analyses, and Sue Bayliss for her assistance with search terms. This study was funded by a non-commercial grant from Grifols Biotherapeutics.
Footnotes
Author contributions
AMT designed the review question; DC was the first reviewer, responsible for all data gathering, extraction and analysis. AMT was the primary independent reviewer, with bias and quality of data extraction reviewed by MR, MK and EVL. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
AMT has received honoraria, research grants or educational grants from or acted as an investigator in trials for Boehringer Ingelheim, Novartis, Chiesi, GSK, AstraZeneca and Pfizer. The authors report no other conflicts of interest in this work.
References
- 1.Turner AM, Tamasi L, Schleich F, et al. Clinically relevant subgroups in COPD and asthma. Eur Respir Rev. 2015;24(136):283–298. doi: 10.1183/16000617.00009014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruban RH, Meziane MA, Zerhouni EA, et al. High resolution computed tomography of inflation-fixed lungs. Pathologic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis. 1987;136(4):935–940. doi: 10.1164/ajrccm/136.4.935. [DOI] [PubMed] [Google Scholar]
- 3.Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170(11):1172–1178. doi: 10.1164/rccm.200406-761OC. [DOI] [PubMed] [Google Scholar]
- 4.Müller NL, Staples CA, Miller RR, Abboud RT. “Density Mask”. An objective method to quantitate emphysema using computed-tomography. Chest. 1988;94(4):782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 5.Diaz AA, Valim C, Yamashiro T, et al. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138(4):880–887. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camiciottoli G, Bartolucci M, Maluccio NM, et al. Spirometrically gated high-resolution CT findings in COPD: lung attenuation vs lung function and dyspnea severity. Chest. 2006;129(3):558–564. doi: 10.1378/chest.129.3.558. [DOI] [PubMed] [Google Scholar]
- 7.Gierada DS, Bierhals AJ, Choong CK, et al. Effects of CT section thickness and reconstruction kernel on emphysema quantification relationship to the magnitude of the CT emphysema index. Acad Radiol. 2010;17(2):146–156. doi: 10.1016/j.acra.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uppaluri R, Mitsa T, Sonka M, Hoffman EA, McLennan G. Quantification of pulmonary emphysema from lung computed tomography images. Am J Respir Crit Care Med. 1997;156(1):248–254. doi: 10.1164/ajrccm.156.1.9606093. [DOI] [PubMed] [Google Scholar]
- 9.Dirksen A, Dijkman JH, Madsen F, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1468–1472. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 10.Crapo J, S E. COPDGene. COPD genetic epidemiology. 2014. [Accessed January 08, 2018]. Available from: www.copdgene.org.
- 11.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 12.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2 Group QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 13.Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 14.Field AP. Is the meta-analysis of correlation coefficients accurate when population correlations vary? Psychol Methods. 2005;10(4):444–467. doi: 10.1037/1082-989X.10.4.444. [DOI] [PubMed] [Google Scholar]
- 15.Park TS, Lee JS, Seo JB, et al. KOLD Study Group Study design and outcomes of Korean Obstructive Lung Disease (KOLD) cohort study. Tuberc Respir Dis (Seoul) 2014;76(4):169–174. doi: 10.4046/trd.2014.76.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SS, Seo JB, Lee HY, et al. Chronic obstructive pulmonary disease: lobe-based visual assessment of volumetric CT by using standard images-comparison with quantitative CT and pulmonary function test in the COPDGene study. Radiology. 2013;266(2):626–635. doi: 10.1148/radiol.12120385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed Hoesein FA, de Jong PA, Lammers JW, et al. Contribution of CT quantified emphysema, air trapping and airway wall thickness on pulmonary function in male smokers with and without COPD. COPD. 2014;11(5):503–509. doi: 10.3109/15412555.2014.933952. [DOI] [PubMed] [Google Scholar]
- 18.Hong Y, Chae EJ, Seo JB, et al. Contributors of the severity of airflow limitation in COPD patients. Tuberc Respir Dis. 2012;72(1):8–14. [Google Scholar]
- 19.Aziz ZA, Wells AU, Desai SR, et al. Functional impairment in emphysema: contribution of airway abnormalities and distribution of parenchymal disease. AJR Am J Roentgenol. 2005;185(6):1509–1515. doi: 10.2214/AJR.04.1578. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed Hoesein FA, van Rikxoort E, van Ginneken B, et al. Computed tomography-quantified emphysema distribution is associated with lung function decline. Eur Respir J. 2012;40(4):844–850. doi: 10.1183/09031936.00186311. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax. 2011;66(9):782–787. doi: 10.1136/thx.2010.145995. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed Hoesein FA, de Jong PA, Lammers JW, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45(3):644–651. doi: 10.1183/09031936.00020714. [DOI] [PubMed] [Google Scholar]
- 23.Stolk J, Ng WH, Bakker ME, et al. Correlation between annual change in health status and computer tomography derived lung density in subjects with alpha1-antitrypsin deficiency. Thorax. 2003;58(12):1027–1030. doi: 10.1136/thorax.58.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowson LJ, Guest PJ, Hill SL, Holder RL, Stockley RA. High-resolution computed tomography scanning in alpha1-antitrypsin deficiency: relationship to lung function and health status. Eur Respir J. 2001;17(6):1097–1104. doi: 10.1183/09031936.01.00056501. [DOI] [PubMed] [Google Scholar]
- 25.Barjaktarevic I, Springmeyer S, Gonzalez X, Sirokman W, Coxson HO, Cooper CB. Diffusing capacity for carbon monoxide correlates best with tissue volume from quantitative CT scanning analysis. Chest. 2015;147(6):1485–1493. doi: 10.1378/chest.14-1693. [DOI] [PubMed] [Google Scholar]
- 26.Motohashi N, Kimura K, Ishii T, et al. Emphysema on imaging is associated with quality of life in elderly patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int. 2010;10(1):17–24. doi: 10.1111/j.1447-0594.2009.00549.x. [DOI] [PubMed] [Google Scholar]
- 27.de Torres JP, Bastarrika G, Zagaceta J, et al. Emphysema presence, severity, and distribution has little impact on the clinical presentation of a cohort of patients with mild to moderate COPD. Chest. 2011;139(1):36–42. doi: 10.1378/chest.10-0984. [DOI] [PubMed] [Google Scholar]
- 28.Martinez CH, Chen YH, Westgate PM, et al. COPDGene Investigators Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez FJ, Curtis JL, Sciurba F, et al. National Emphysema Treatment Trial Research Group Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med. 2007;176(3):243–252. doi: 10.1164/rccm.200606-828OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gietema HA, Edwards LD, Coxson HO, Bakke PS, ECLIPSE Investigators Impact of emphysema and airway wall thickness on quality of life in smoking-related COPD. Respir Med. 2013;107(8):1201–1209. doi: 10.1016/j.rmed.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Camiciottoli G, Bigazzi F, Bartolucci M, et al. BODE-index, modified BODE-index and ADO-score in chronic obstructive pulmonary disease: relationship with COPD phenotypes and CT lung density changes. COPD. 2012;9(3):297–304. doi: 10.3109/15412555.2012.661000. [DOI] [PubMed] [Google Scholar]
- 32.Haruna A, Oga T, Muro S, et al. Relationship between peripheral airway function and patient-reported outcomes in COPD: a cross-sectional study. BMC Pulm Med. 2010;10:10. doi: 10.1186/1471-2466-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138(3):635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 34.Martinez FJ, Foster G, Curtis JL, et al. NETT Research Group Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawkins P, Wood A, Nightingale P, Stockley R. Mortality in alpha-1-antitrypsin deficiency in the United Kingdom. Respir Med. 2009;103(10):1540–1547. doi: 10.1016/j.rmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Yoo JW, Hong Y, Seo JB, et al. Comparison of clinico-physiologic and CT imaging risk factors for COPD exacerbation. J Korean Med Sci. 2011;26(12):1606–1612. doi: 10.3346/jkms.2011.26.12.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng T, Wan HY, Cheng QJ, et al. Obvious emphysema on computed tomography during an acute exacerbation of chronic obstructive pulmonary disease predicts a poor prognosis. Intern Med J. 2015;45(5):517–526. doi: 10.1111/imj.12723. [DOI] [PubMed] [Google Scholar]
- 38.Vijayasaratha K, Stockley RA. Relationship between frequency, length, and treatment outcome of exacerbations to baseline lung function and lung density in alpha-1 antitrypsin-deficient COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:789–796. doi: 10.2147/COPD.S31797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAllister DA, Ahmed FS, Austin JH, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9(4):e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han MK, Kazerooni EA, Lynch DA, et al. COPDGene Investigators Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman KR, Burdon JG, Piitulainen E, et al. RAPID Trial Study Group Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 42.Dirksen A, Piitulainen E, Parr DG, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J. 2009;33(6):1345–1353. doi: 10.1183/09031936.00159408. [DOI] [PubMed] [Google Scholar]
- 43.Parr DG, Dirksen A, Piitulainen E, Deng C, Wencker M, Stockley RA. Exploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiency. Respir Res. 2009;10:75. doi: 10.1186/1465-9921-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgar RG, Patel M, Bayliss S, Crossley D, Sapey E, Turner AM. Treatment of lung disease in alpha-1 antitrypsin deficiency: a systematic review. Int J Chron Obstruct Pulmon Dis. 2017;12:1295–1308. doi: 10.2147/COPD.S130440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roth MD, Connett JE, D’Armiento JM, et al. FORTE Study Investigators Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130(5):1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 46.Stolk J, Cooper BG, Stoel B, et al. Retinoid treatment of Emphysema in patients on the Alpha-1 International Registry. The REPAIR study: study design, methodology and quality control of study assessments. Ther Adv Respir Dis. 2010;4(6):319–332. doi: 10.1177/1753465810379617. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Lee YK, Kim EK, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med. 2010;104(4):542–549. doi: 10.1016/j.rmed.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Lee JS, Huh JW, Chae EJ, et al. Predictors of pulmonary function response to treatment with salmeterol/fluticasone in patients with chronic obstructive pulmonary disease. J Korean Med Sci. 2011;26(3):379–385. doi: 10.3346/jkms.2011.26.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaker SB, Dirksen A, Ulrik CS, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD. 2009;6(2):104–111. doi: 10.1080/15412550902772593. [DOI] [PubMed] [Google Scholar]
- 50.Washko GR, Criner GJ, Mohsenifar Z, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD. 2008;5(3):177–186. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 51.Gøtzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev. 2010;(7):CD007851. doi: 10.1002/14651858.CD007851.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging. 2011;11(Spec No A):S79–S84. doi: 10.1102/1470-7330.2011.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wise RA, Drummond MB. The role of NETT in emphysema research. Proc Am Thorac Soc. 2008;5(4):385–392. doi: 10.1513/pats.200709-153ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemerink GJ, Kruize HH, Lamers RJ, van Engelshoven JM. Density resolution in quantitative computed tomography of foam and lung. Med Phys. 1996;23(10):1697–1708. doi: 10.1118/1.597757. [DOI] [PubMed] [Google Scholar]
- 56.Kemerink GJ, Kruize HH, Lamers RJ, van Engelshoven JM. Density resolution in quantitative computed tomography of foam and lung. Med Phys. 1996;23(10):1697–1708. doi: 10.1118/1.597757. [DOI] [PubMed] [Google Scholar]
- 57.Wielputz MO, Bardarova D, Weinheimer O, et al. Variation of densitometry on computed tomography in COPD – influence of different software tools. PLoS One. 2014;9(11):e112898. doi: 10.1371/journal.pone.0112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim EY, Seo JB, Lee HJ, et al. Detailed analysis of the density change on chest CT of COPD using non-rigid registration of inspiration/expiration CT scans. Eur Radiol. 2015;25(2):541–549. doi: 10.1007/s00330-014-3418-0. [DOI] [PubMed] [Google Scholar]
- 59.Nambu A, Zach J, Schroeder J, et al. Relationships between diffusing capacity for carbon monoxide (DLCO), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur J Radiol. 2015;84(5):980–985. doi: 10.1016/j.ejrad.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Chae EJ, Seo JB, Song JW, et al. Slope of emphysema index: an objective descriptor of regional heterogeneity of emphysema and an independent determinant of pulmonary function. AJR Am J Roentgenol. 2010;194(3):W248–W255. doi: 10.2214/AJR.09.2672. [DOI] [PubMed] [Google Scholar]
- 61.Diaz S, Casselbrant I, Piitulainen E, et al. Validity of apparent diffusion coefficient hyperpolarized 3He-MRI using MSCT and pulmonary function tests as references. Eur J Radiol. 2008;71(2):257–263. doi: 10.1016/j.ejrad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 62.O’Donnell RA, Peebles C, Ward JA, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59(10):837–842. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gevenois PA, De Vuyst P, Sy M, et al. Pulmonary emphysema: quantitative CT during expiration. Radiology. 1996;199(3):825–829. doi: 10.1148/radiology.199.3.8638012. [DOI] [PubMed] [Google Scholar]
- 64.Baldi S, Miniati M, Bellina CR, et al. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(4):585–589. doi: 10.1164/ajrccm.164.4.2010066. [DOI] [PubMed] [Google Scholar]
- 65.Saitoh T, Koba H, Shijubo N, Tanaka H, Sugaya F. Lobar distribution of emphysema in computed tomographic densitometric analysis. Invest Radiol. 2000;35(4):235–243. doi: 10.1097/00004424-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201(3):W460–W470. doi: 10.2214/AJR.12.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bastarrika G, Wisnivesky JP, Pueyo JC, Diaz L, Arraiza M, Villanueva A, et al. Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. 2009;24(3):206–211. doi: 10.1097/RTI.0b013e3181a65263. [DOI] [PubMed] [Google Scholar]
- 68.Paoletti M, Cestelli L, Bigazzi F, Camiciottoli G, Pistolesi M. Chronic obstructive pulmonary disease: pulmonary function and CT lung attenuation do not show linear correlation. Radiology. 2015;276(2):571–578. doi: 10.1148/radiol.2015141769. [DOI] [PubMed] [Google Scholar]
- 69.Orlandi I, Moroni C, Camiciottoli G, et al. Chronic obstructive pulmonary disease: thin-section CT measurement of airway wall thickness and lung attenuation. Radiology. 2005;234(2):604–610. doi: 10.1148/radiol.2342040013. [DOI] [PubMed] [Google Scholar]
- 70.Nishio M, Matsumoto S, Koyama H, Ohno Y, Sugimura K. Airflow limitation in chronic obstructive pulmonary disease: ratio and difference of percentage of low-attenuation lung regions in paired inspiratory/expiratory computed tomography. Acad Radiol. 2014;21(10):1262–1267. doi: 10.1016/j.acra.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 71.Shaker SB, Maltbaek N, Brand P, Haeussermann S, Dirksen A. Quantitative computed tomography and aerosol morphometry in COPD and alpha1-antitrypsin deficiency. Eur Respir J. 2005;25(1):23–30. doi: 10.1183/09031936.04.00075304. [DOI] [PubMed] [Google Scholar]
- 72.Bernspang E, Diaz S, Stoel B, Wollmer P, Sveger T, Piitulainen E. CT lung densitometry in young adults with alpha-1-antitrypsin deficiency. Respir Med. 2011;105(1):74–79. doi: 10.1016/j.rmed.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 73.Akira M, Toyokawa K, Inoue Y, Arai T. Quantitative CT in chronic obstructive pulmonary disease: inspiratory and expiratory assessment. AJR Am J Roentgenol. 2009;192(1):267–272. doi: 10.2214/AJR.07.3953. [DOI] [PubMed] [Google Scholar]