Figure 3.
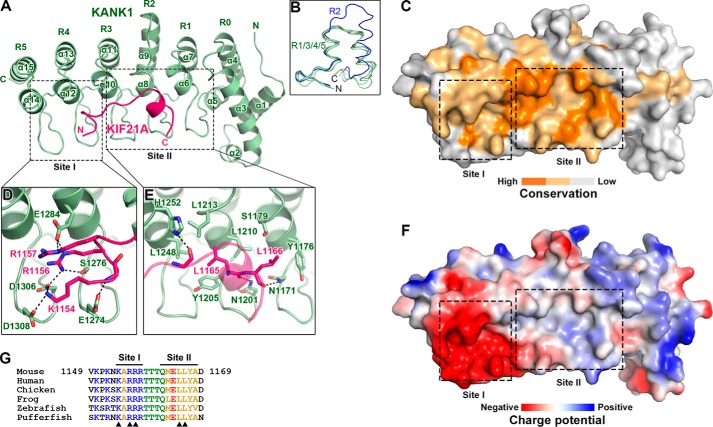
Structural characterization of the KANK1-KIF21A interaction. A, ribbon representation of the heterodimeric complex with KANK1_ANKRD in green and KIF21A_H in pink. This color coding scheme is used throughout the remaining figures unless otherwise indicated. B, structural comparison of the five ANK repeats in the ANKRD domain of KANK1. C, surface conservation of the ANKRD. Based on the sequence alignment shown in Fig. 2, identical and similar residues are colored in orange and light orange, respectively. D and E, detailed interactions in site-I (D) and site-II (E). Interface residues are shown in stick representation. Hydrogen bonds and salt bridges are indicated by dashed lines. F, charge potential surface of KANK1 reveals a negatively charged pocket in site-I. G, amino acid sequence alignment of KIF21A_H in vertebrates. Residues directly involved in the site-I and site-II interactions are indicated by triangles.