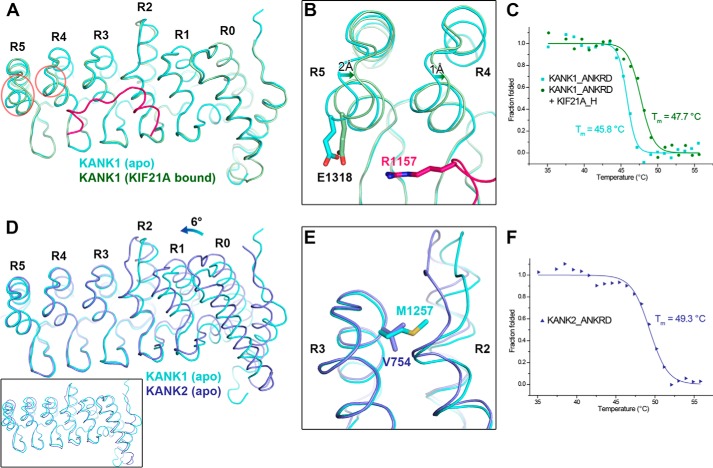
Figure 6.
Structural comparison of the ANKRDs. A, overlap of KANK1_ANKRD in apo form and KIF21A-bound form. Conformational changes in the R4 and R5 repeats are indicated by red circles. B, charge-charge interaction between Glu-1318KANK1 and Arg-1157KIF21A. C, CD-based thermal denaturation analysis of KANK1_ANKRD in the presence and absence of the KIF21A_H peptide. D, overlap of the C-terminal ANK repeats (R3–5) of the ANKRDs in KANK1 and KANK2 (Protein Data Bank code 4HBD) showing the rotational change of the N-terminal region (R0–2). An overall structural comparison of these two ANKRD apo structures is shown in the inset. E, comparison of the R2/3 interfaces of the two structures in D. F, thermal denaturation analysis of KANK2_ANKRD.